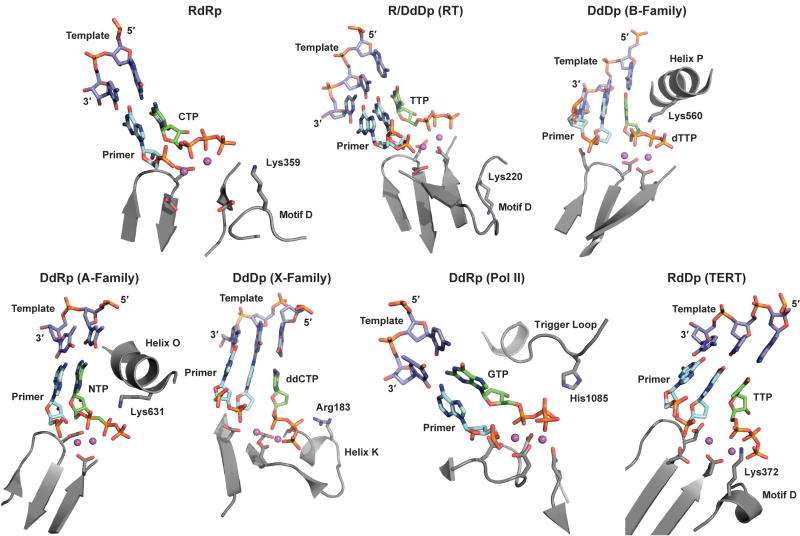
Figure 2. Interactions of NTP in the active sites of various polymerase families.
In all ternary complexes, the NTP (green C-atoms), primer/template (cyan and purple C-atoms, respectively), and key active site residues (grey C-atoms) are shown as sticks; the metal ions are displayed as spheres colored in magenta. RdRp: The RdRp from PV (pdb 1RA6) 19 with primer/template and CTP bound to the active site in the presence of two Mn2+ ions. Primer-template, CTP and Mn2+ ions were extracted from the structural complex of Norovirus (NV) polymerase (pdb 3BS0) 22 and modeled into the active site of PV RdRp. R/DdDp (RT): HIV RT with TTP bound to the active site in the presence of two Mg2+ ions (pdb 1RTD) 14. DdDp (B-Family): The RB69 DNA polymerase with the dTTP nucleotide bound in the presence of Ca2+ ions (pdb 1IG9) 12. DdRp (A-Family): The T7 RNA polymerase with the modified nucleotide, αβ-methylene-ATP bound in the presence of Mg2+ (pdb 1S76) 21. DdDp (X-Family): Pol β polymerase with the ddCTP bound to the active site in the presence of Mg2+ ions (pdb 1BPY) 50. DdRp (Pol II): Multi-subunit RNA polymerase II from yeast (pdb 2E2H) 31 in complex with nucleic acids and GTP substrate in the presence of Mg2+. RdDp (TERT): The catalytic subunit of telomerase with TTP bound to the active site in the presence of two Mg2+ ions (pdb 3DU5) 25. Primer-template, TTP and Mg2+ ions were extracted from the structural complex of HIV RT (1RTD) 14 and modeled into the active site of TERT as described previously 25. Residues equivalent to Lys359 of PV RdRp predicted to function as general acids, are labeled. The designations of the structural elements on which the general acid is located are also indicated explicitly.