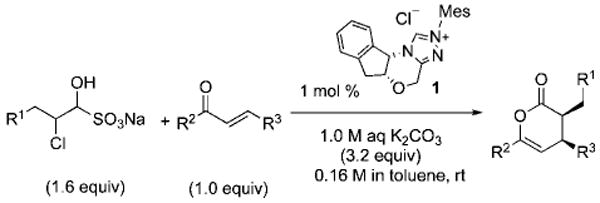
Table 3.
NHC-Catalyzed Biphasic Diels–Alder Reactions of Chloroaldehyde Bisulfite Saltsa
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R1= | R2= | R3= | t / h | product | yield / %b | ee / %c |
| 1 | Ph 9 | Ph | C02Et 3b | 6.0 | 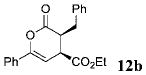 |
98 | 99 |
| 2 | Ph 9 | p-Br-C6H4 | C02Et 3c | 2.0 | 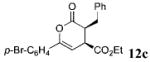 |
84 | >99 |
| 3 | Ph 9 | p-OMe-C6H4 | C02Et 3d | 15.0 | 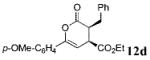 |
55 | >99 |
| 4 | Ph 9 | c-Hex | C02Et 3e | 10.0 | 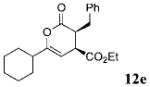 |
70 | 91 |
| 5 | Ph 9 | Me | C02Et 3f | 6.0 | 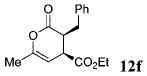 |
65 | 99 |
| 6 | c-Hex 10 | Ph | C02Et 3b | 15.5 |
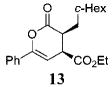
|
59 | >99 |
| 7 | n-C9H1911 | Ph | C02Et 3b | 4.5 |
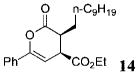
|
74 | >99 |
| 8 | Ph 9 | C02Et | p-Tol 4a | 5.5 |
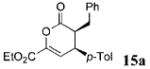
|
73 | >99 |
| 9 | Ph 9 | C02Et | n-Pr 4b | 5.0 |
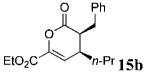
|
78 | >99 |
All reactions were performed at 0.16 M in toluene with 1.6 equiv of chloroaldehyde bisulfite adduct, 1.0 equiv of oxodiene, 1 mol % of 1, and 3.2 equiv of 1.0 M aq K2CO3. In all cases, only one diastereomer was detected in unpurified reaction mixtures.
Isolated yield after chromatography.
Determined by HPLC or SFC analysis.
