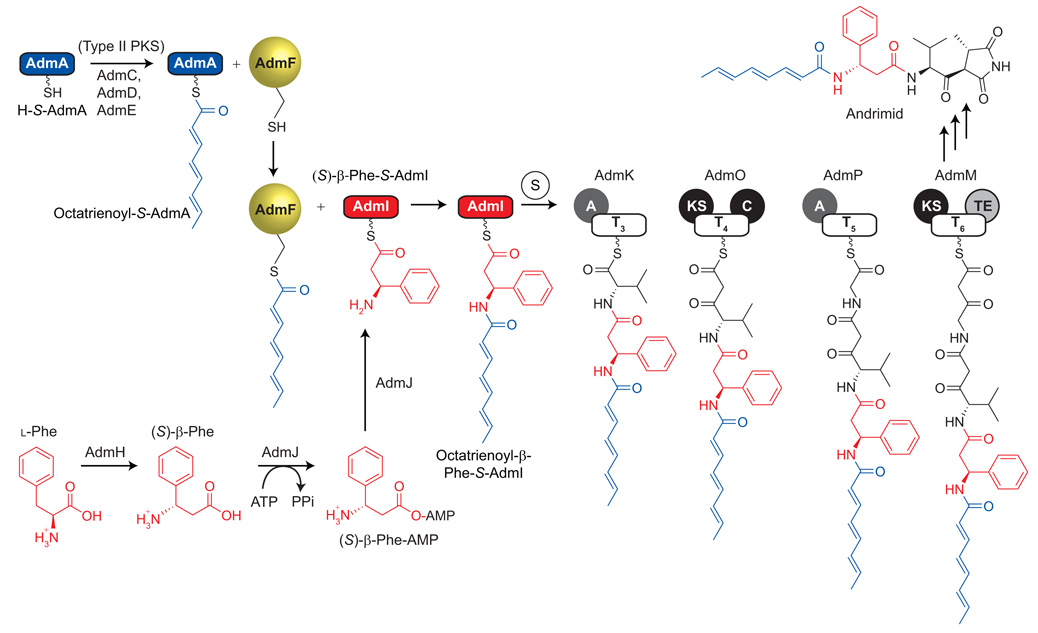
Figure 1.
Highly dissociated biosynthetic pathway of andrimid. The early biosynthetic steps are emphasized. The polyunsaturated acyl chain is installed onto the phosphopantetheinyl (Ppant) arm of holo-AdmA (HS-AdmA) through the actions of a type II PKS. The resulting octatrienoyl-S-AdmA (blue) serves as substrate for the self-acylating transglutaminase homologue AdmF (yellow). (S)-β-Phe is synthesized from l-Phe by the MIO-containing aminomutase AdmH and is activated to the corresponding AMP-ester by the A domain AdmJ. AdmJ then installs (S)-β-Phe onto the Ppant arm of holo-AdmI (HS-AdmI), leading to the formation of (S)-β-Phe-S-AdmI (red). AdmF catalyzes the formation of an isopeptide bond between the octatrienoyl chain and the amine group of α-phenylalanine through the acylation of its active site cysteine (Cys90). The hybrid biosynthetic pathway proceeds through six T domains before final tailoring leads to the maturation of the final andrimid antibiotic.