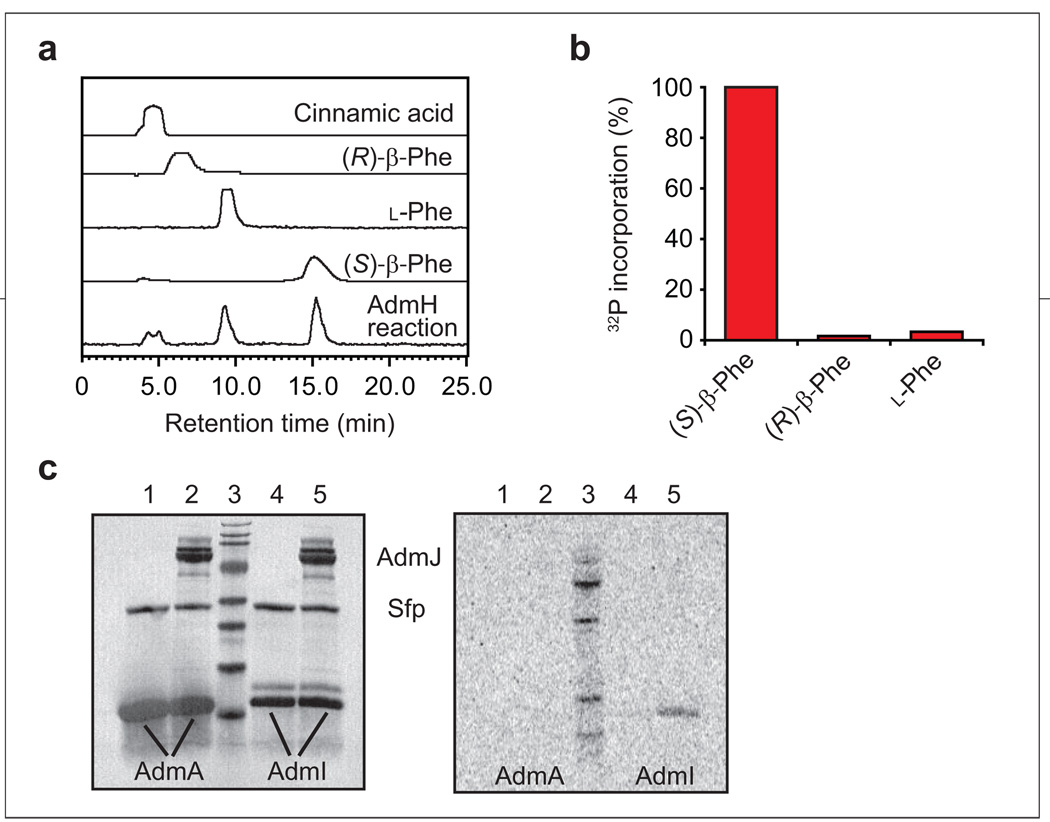
Figure 2.
Early gatekeepers in the biosynthesis and loading of the phenylalanine building block. a) HPLC traces of the AdmH aminomutase reaction. The AdmH reaction was carried out for 90 min at 30 °C using [14C]-labeled l-Phe as substrate. Non-labeled cinnamic acid, (S)-β-Phe, and (R)-β-Phe and [14C]-labeled l-Phe were used as standards. The AdmH reaction and standards were analyzed by chiral HPLC using an Astec Chirobiotic T2 column and a 9:1 (methanol/water) solvent system. The AdmH reaction produced (S)-β-Phe and cinnamic acid. b) Adenylation of phenyla-nine isomers by AdmJ. ATP/pyrophosphate assay carried out using (S)-β-Phe, (R)-β-Phe, and l-Phe. c) AdmJ-catalyzed loading of (S)-β-Phe on AdmI and AdmA. [14C]-labeled (S)-β-Phe was incubated with holo-AdmA in the absence and presence of AdmJ (lanes 1 and 2, respectively). [14C]-labeled (S)-β-Phe was incubated with holo-AdmI in the absence and presence of AdmJ (lanes 4 and 5, respectively). All reactions were quenched after 60 min of incubation at 30 °C. Left, SDS–PAGE; right, autoradiogram.