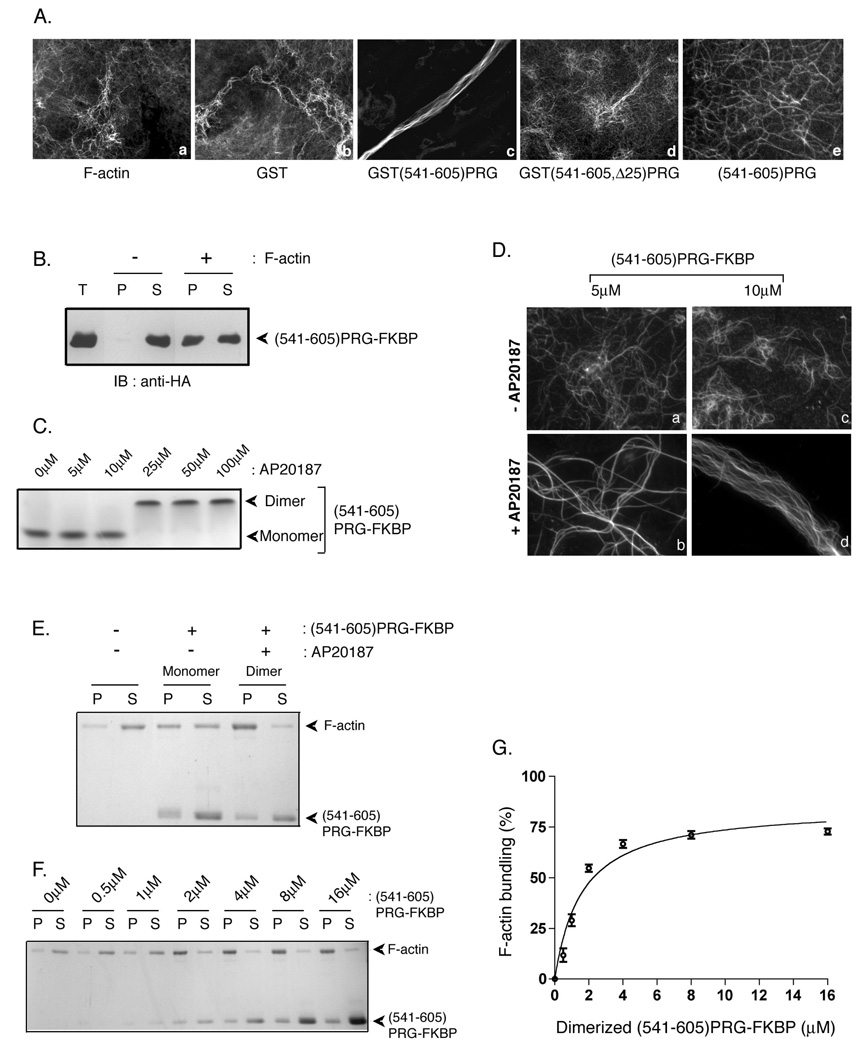
Figure 6. PRG induces F-actin bundling in vitro.
(A) F-actin (1.2 µM) was incubated alone (a) or with 7 µM GST (b), GST(541–605)PRG (c), GST(541–605, Δ25)PRG (d), or (541–605)PRG in which GST was removed by proteolysis (e) for 30 min on ice. After staining with phalloidin, actin filaments and bundles were observed by fluorescence microscopy. (B) (541–605)PRG-FKBP (GST was removed by proteolysis) incubated with or without freshly polymerized actin was subjected to high speed co-sedimentation assay at 160,000g for 90 min. Equal aliquots of pellet and supernatant fractions along with total protein (T) were immunoblotted using anti-HA antibody to detect (541–605)PRG-FKBP. (C) 25 µM (541–605)PRG-FKBP was incubated with variable concentrations (5–100 µM) of AP20187 for 15 min at room temperature. Samples were subject to native gel electrophoresis followed by colloidal blue staining. 25 µM (541–605)PRG-FKBP was used to observe consistent colloidal blue staining. (D) (541–605)PRG-FKBP (5 µM and 10 µM) treated without (a and c) and with (b and d) AP20187 was incubated with 1.2 µM freshly polymerized F-actin. Following incubation, F-actin was stained with phalloidin and observed by fluorescence microscopy. (E) 2 µM monomeric or dimeric (541–605)PRG-FKBP was incubated with 1 µM freshly polymerized F-actin, and samples were centrifuged at 10,000g for 30 min. Equal amounts of pellet (P) and supernatant (S) fractions were resolved by SDS-PAGE and stained using colloidal blue. (F) To quantitate F-actin bundling activity of dimerized (541–605)PRG-FKBP, 0, 0.5, 1, 2, 4, 8 or 16 µM of dimerized (541–605)PRG-FKBP was incubated with 1 µM of F-actin. Samples were then centrifuged at 10,000g. amounts of pellet (P) and supernatant (S) fractions were run on SDS-PAGE and stained with colloidal blue. (G) F-actin in the 10,000g pellet fraction in (F) was quantitated using densitometry. The percentage of F-actin bundled using different amounts of dimerized (541–605)PRG-FKBP were calculated as the percentage recovered in the pellet (P) over total protein (S+P).The data represent the means ± S.D. of three independent experiments.