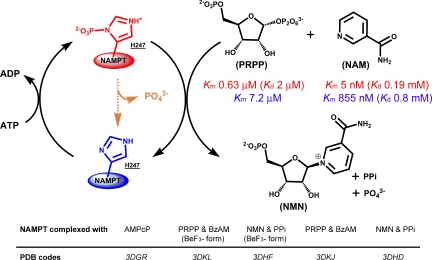
Fig. 1.
Human NAMPT exists in P-H247 (red) and H247 (blue) forms. P-H247 NAMPT is 1,125-fold more active (kcat/Km values) and requires continual hydrolysis of ATP. P-H247 NAMPT has low Km values for NAM and PRPP (red) while H247 NAMPT has higher Km values (blue). NAMPT slowly hydrolyzes ATP (orange) even in the absence of NAM and PRPP. In the presence of ATP, the stoichiometry of NMN:ADP formation is always 1.0 or less, indicating P-H247 hydrolysis on each NAM synthetic cycle. PRPP has a tighter affinity than NAM for P-H247, suggesting a preferential ordered binding of substrates to the enzyme, with PRPP binding first. Five crystallographic structures illustrate the main steps of this complex mechanism.