Table 3.
Substrate scope of Pd-catalyzed enyne cyclization reactionsa.
| Entry | Substrate | Product | Yieldb |
|---|---|---|---|
| 1 | 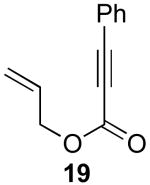 |
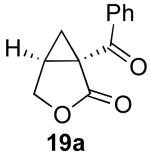 |
79% |
| 2 | 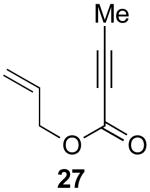 |
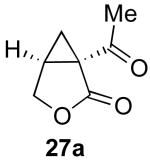 |
55% |
| 3 | 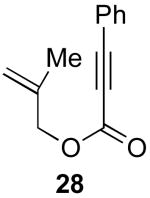 |
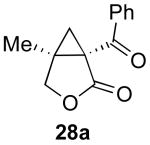 |
78% |
| 4 | 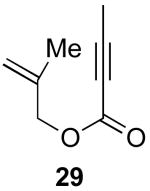 |
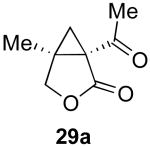 |
66% |
| 5 | 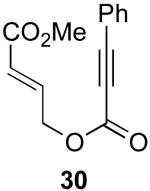 |
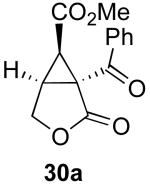 |
70% |
| 6 | 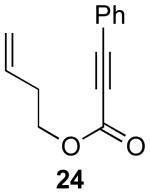 |
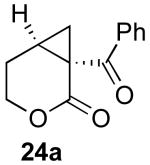 |
55% |
| 7 | 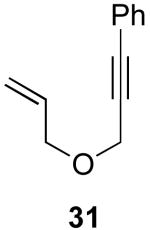 |
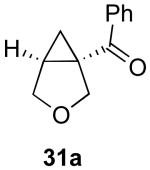 |
48% |
| 8 | 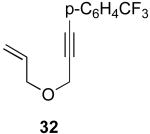 |
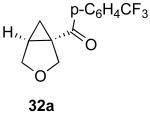 |
41% |
| 9 | 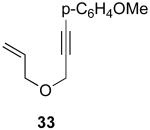 |
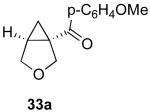 |
44% |
| 10 | 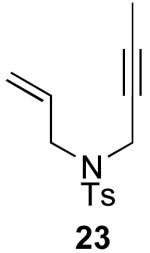 |
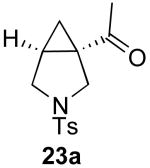 |
71% |
| 11 | 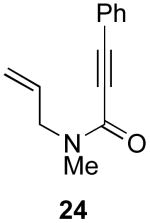 |
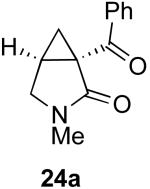 |
47% |
Reaction Conditions: 5 mol % Pd(OAc)2, 0-6 mol % bipy, 1.1-4 equiv PhI(OAc)2, 60-80 °C, 1-16 h.
Isolated yields (average of two runs).
