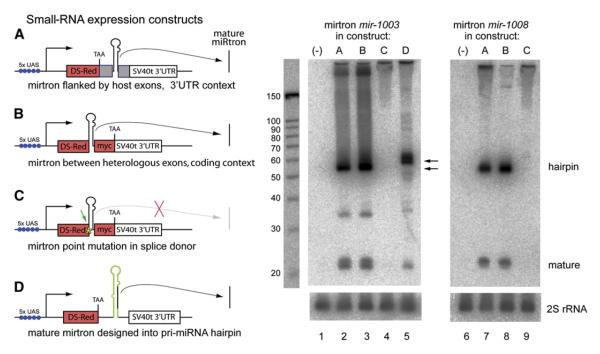
Figure 4. Structure-Function Analysis of Mirtron Biogenesis.
(A)—(D) On the left are four constructs for mirtron expression. In (A), ∼400 nt pri-mirtrons containing their flanking endogenous exons (gray boxes) were cloned into the 3′ UTR of UAS-DsRed. In (B), ∼55-70 nt mirtron hairpins were cloned between coding exons for DsRed and 2xmyc, thereby separating them from endogenous exonic context. In (C), using construct B as a template, a G -> C point mutation in the first base of the 5′ splice donor was introduced (asterisk and arrow). In (D), the mature ∼22 nt mirtron product was substituted into a miRNA precursor based on pri-mir-6-1 and then inserted into the 3′ UTR of UAS-DsRed. On the right, northern analysis of mirtrons cloned into constructs (A)—(D), as depicted on the left, and activated in S2 cells using ub-Gal4. Lanes 2 and 6 show that miR-1003, miR-1008, and their associated mirtron hairpins were efficiently generated from mirtron genomic fragments using construct (A), (compare with lanes 1 and 6 for endogenous mirtron expression; note that the blot has been underexposed relative to blots in Figure 3). miR-1003 and miR-1008 and their mirtron hairpins were also readily expressed from construct B (lanes 3 and 8), demonstrating that endogenous flanking exons are dispensable for entry into the mirtron pathway. Point mutation of the splice donor site demonstrated that splicing is necessary to generate the mirtron hairpin (lanes 4 and 9). Mature miR-1003 could also be expressed by reprogramming a canonical pri-miRNA (lane 5). Since the mir-6-1 hairpin is 63 nt, the hybrid pri-mir-6-1/mir-1003 hairpin is slightly longer than 56-nt-long mirtron for miR-1003 (as indicated by arrows). Blots were stripped and reprobed for 2S rRNA as a loading control.