Table 2.
Formation and Rearrangement of Selenosulfides
| Entry | Thiol | Donor | Donor (% yield) |
Product a,b | Rearrangement yield (equiv of PPh3) |
||
|---|---|---|---|---|---|---|---|
| X = SO3−K+ | X = CN | from X = SO3−K+ | from X = CN | ||||
| 1 | Me(CH2)15SH | 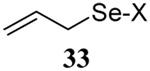 |
56 | 84 | 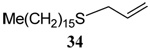 |
74% (0) | 70% (0) |
| 2 | 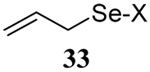 |
56 | 84 | 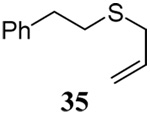 |
70% (0) | 70% (0) | |
| 3 | 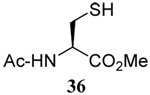 |
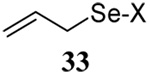 |
56 | 84 | 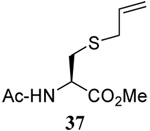 |
61% (2.5) | 80% (0) |
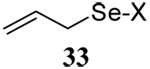
| 4 | 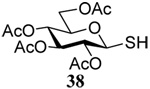 |
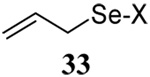 |
56 | 84 | 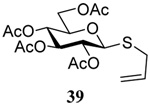 |
50% (2.5)c | 55 (2.5)d |
| 5 |  |
 |
56 | 84 | 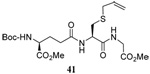 |
58% (2.5) | 75% (0) |
| 6 | 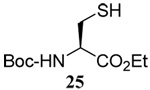 |
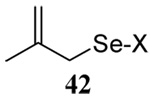 |
58 | 73 | 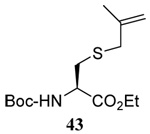 |
62% (2.5) | 68% (0) |
| 7 | 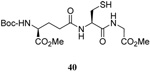 |
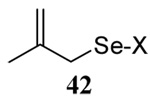 |
58 | 73 | 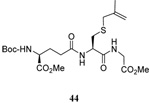 |
60% (2.5) | 71% (0) |
| 8 | 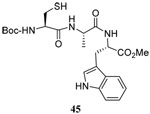 |
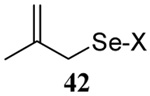 |
58 | 73 | 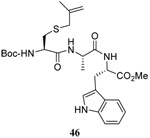 |
60% (2.5) | 65% (2.5) |
| 9 | Me(CH2)7SH | 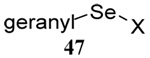 |
40 | 80 |  |
62% (2.5) | 70% (2.5) |
| 10 | 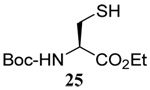 |
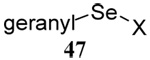 |
40 | 80 | 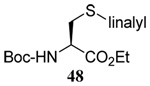 |
46% (2.5) | 66% (2.5) |
| 11 |  |
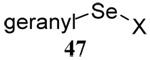 |
40 | 80 | 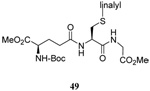 |
43% (2.5) | 55% (2.5) |
| 12 | 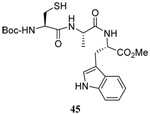 |
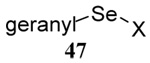 |
40 | 80 | 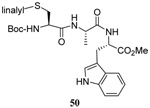 |
51% (2.5) | 50% (2.5) |
| 13 | 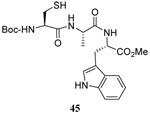 |
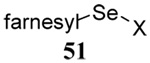 |
35 | 85 | 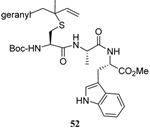 |
47% (2.5) | 40% (2.5) |
| 14 | 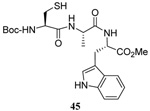 |
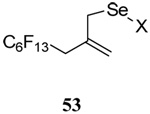 |
28 | 75 |  |
31% (2.5) | 65% (2.5) |
Unless otherwise stated all reactions were conducted in MeOH
at room temperature
T = 65 °C
CDCl3 was used as a solvent at T = 65 °C.
