Table 5.
Desulfurative Rearrangement of Allylic Disulfides in the Absence of Phosphine
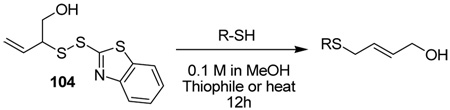 | |||||
|---|---|---|---|---|---|
| Entry | Thiol | Product | Method |
||
| PPh3 % yield, E/Z | Piperidine % yield, E/Z | Heat % yield E/Z | |||
| 1 | 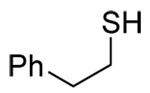 |
45 E only |
62 2.6:1 |
78 E only |
|
| 105 | |||||
| 2 | 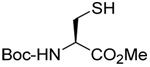 |
- | - | 67 E only |
|
| 83 | 106 | ||||
| 3 | 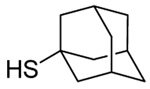 |
- | - | 68 E only |
|
| 107 | |||||
| 4 | Me(CH2)7SH | - | - | 75 E only |
|
| 108 | |||||
| 5 | 4-Me-C6H4-SH | 18 E only |
66 2.5:1 |
66 E only |
|
| 109 | |||||
| 6 | PhSH | - | - | 63 E only |
|
| 110 | |||||
| 7 | 4-Cl-C6H4-SH | - | - | 62 E only |
|
| 111 | |||||
| 8 | 4-NO2-C6H4SH | - | - | 20 E only |
|
| 112 | |||||
| 9 | 2-PySH | - | - | 45 E only |
|
| 113 | |||||
| 10 | 2-HO-C6H4SH | - | - | 57 E only |
|
| 114 | |||||
