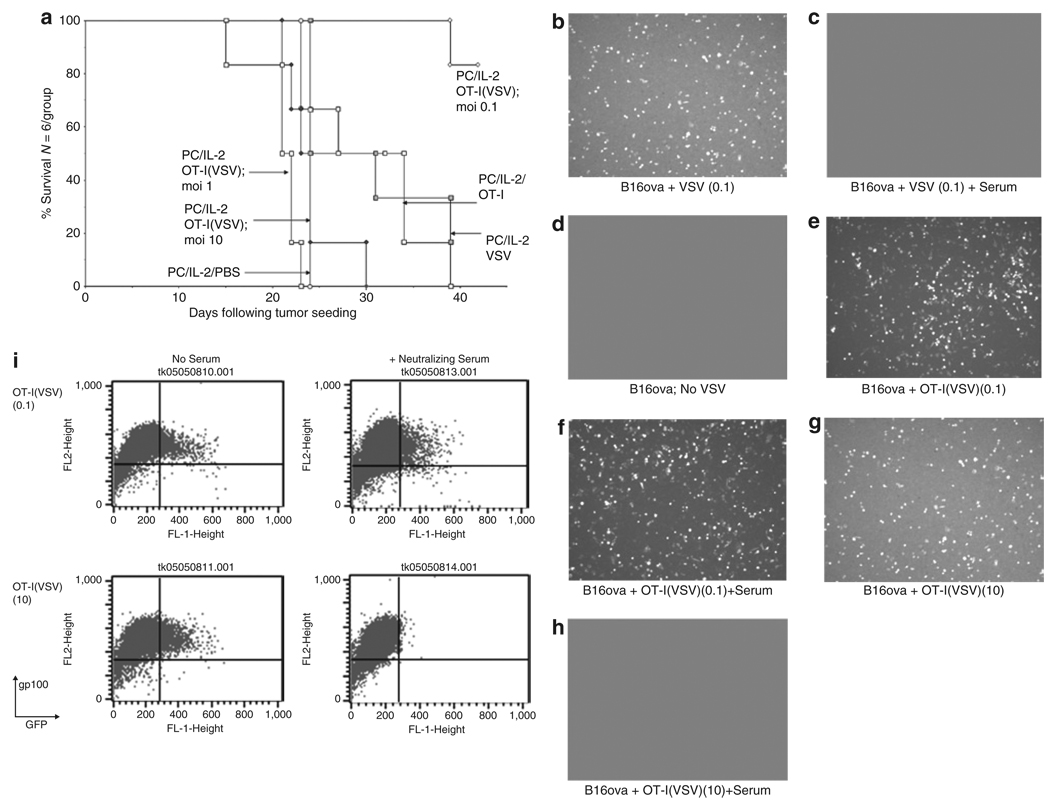
Figure 5. OT-I(VSV) are therapeutically effective in virus-immune mice at low MOI of virus loading.
(a) C57Bl/6 mice were vaccinated i.v. with 5 × 108 pfu VSV which induces high levels of both anti-VSV neutralizing antibody and anti-VSV T cells.32 Twenty-one days later, these virus-immune mice were seeded with s.c. B16ova tumors. Ten days later, all mice received an intraperitoneal injection of anti-CD25 antibody PC-61 followed 24 hours later by i.p. injections of rhIL-2 (75,000 U/injection) three times a day for 3 days. On the fourth day, a single further injection of IL-2 was given. Two and twenty-four hours after this last injection of IL-2, mice received an intravenous injection of PBS; or 107 pfu of VSV-GFP (VSV); or 1 × 106 untreated OT-I (OT-I); or 1 × 106 OT-I loaded with VSV at an MOI of 0.1, 1, or 10 (OT-I(VSV) moi 0.1, 1, or 10). Survival with time following initial tumor seeding is shown. (b–i) 2 × 104 pfu of VSV, 2 × 105 OT-I T cells, or 2 × 105 OT-I loaded with VSV at a MOI of 0.1 or 10 (OT-I(VSV) moi 0.1 or 10) were incubated at 37 °C for 1 hour in the presence of 100 µl of mouse control, or anti-VSV (neutralizing titer 1:2560), serum. Virus or cells were then added to 2 × 105 B16ova cells plated 24 hours previously. After 12 hours, cultures were examined for GFP expression by fluorescence microscopy (b–h) or by flow cytometry (i) for GFP and gp100, a melanoma-specific antigen32 not expressed by OT-I T cells. In all samples, <0.1% of the total cell populations analyzed were GFP+ve, gp100−ve, confirming our previous observation that VSV-GFP infection of OT-I T cells is minimal.31 Therefore, fluorescence shown in b–h represents infection of B16ova target cells by VSV-GFP rather than infected OT-I. At later time points after infection (>24 hours), B16ova cultures showed extensive cytotoxicity associated with VSV oncolysis in cultures b, e, f, and g. Minimal VSV-associated cytotoxicity was observed in cultures c, d, and h.