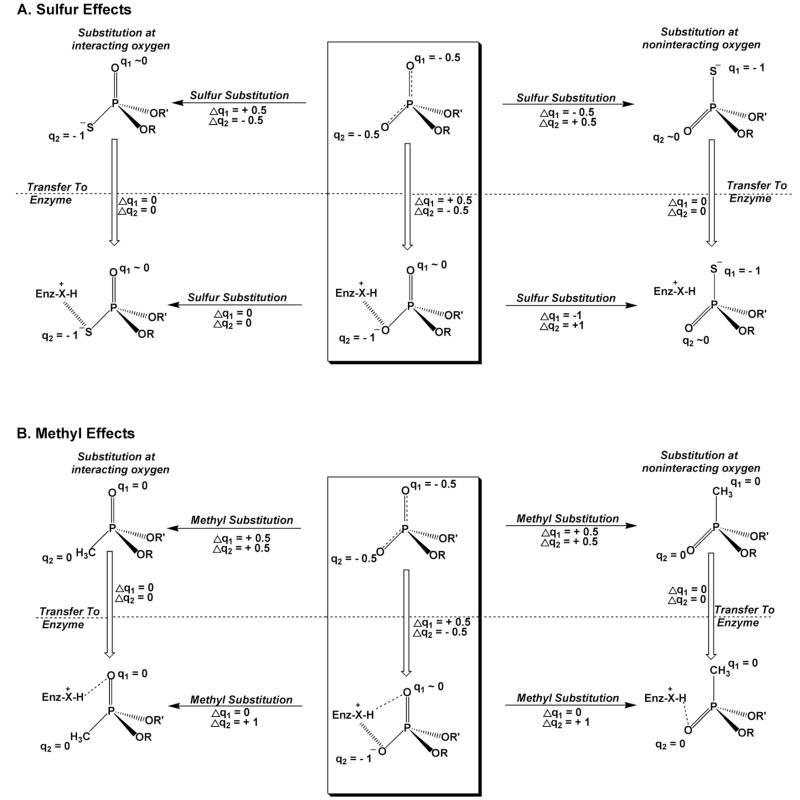
Figure 5.
Possible changes in charge of the nonbridging phosphate diester atoms upon substitution with sulfur or a methyl group in solution and in an enzyme active site. The charges shown on the atoms are approximate and are merely used for illustrating the possible effects. Two cases are depicted: one where the substituent interacts with an enzyme group (left), and one where the substituent does not interact (right). The effects on hydrogen bonding and electrostatics in an enzyme active site are depicted. (A) The center box shows the charge distribution of a phosphate diester in solution, and upon transfer to an enzyme active site (vertical arrow). An enzyme cationic group (X-H+) is depicted that can serve as a charged hydrogen bond donor (hashed lines). Thus, the change in charge (Δq2) for the interacting oxygen upon transfer is −0.5 whereas the other noninteracting oxygen shows a +0.5 change (Δq1). Sulfur substitution for oxygen leads to accumulation of a full negative charge on S for both sulfur diastereomers (horizontal arrows, top). Unlike oxygen, a thio substituted position that interacts with a cationic enzyme group will feel little change in charge (Δq2 or Δq1), because sulfur is already carrying a full negative charge in solution (left vertical arrow). A similar analysis is shown for a noninteracting position (top, right vertical arrow). The net result is that nonbridging thio substitution in the context of the enzyme active site (horizontal arrows, bottom panel A) produces no changes in charge for the directly acting case (right), and large changes in charge for the noninteracting substitution. (B) An analogous scenario for methyl substitution.