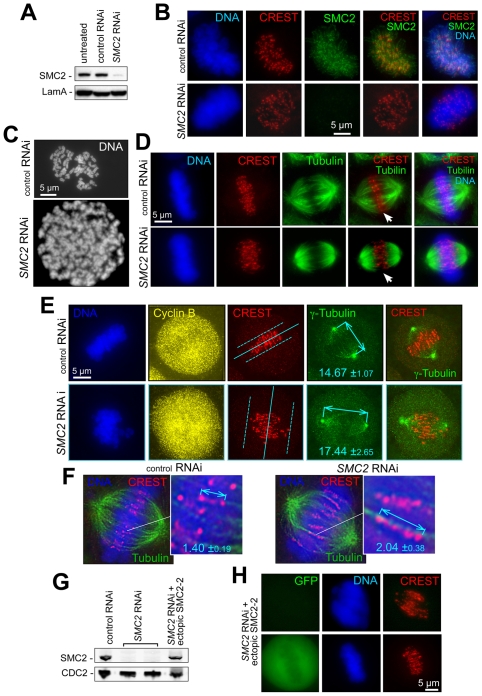
Figure 1. Depletion of condensins 1 and 2 disorganizes metaphase chromosome alignment and centromere structure.
A) SMC2 depletion by RNAi. Immunoblotting with specific condensin antibodies (see Methods) four days after siRNA transfection; Laminin A – loading control. Indirect immunofluorescence images of fixed preparations of HeLa cells transfected with SMC2 siRNA or mismatched siRNA (control RNAi) and stained with DAPI (DNA) and anti-SMC2 antibodies four days after transfection. B) Disorganization of metaphase alignment in condensin-depleted cells. Fixed preparations of HeLa cells transfected with SMC2 siRNA or mismatched siRNA (control RNAi) were stained with DAPI (DNA), anti-centromere (CREST) and anti-SMC2 antibodies 4 days after transfection. SMC2 depletion always produced a diffuse metaphase plate, accompanied by the loss of visual separation between sister centromeres and their delocalization from the spindle mid-zone (Fig. 1D–1F). C) Chromosome condensation defects in SMC2-depleted cells. Chromosome morphology was analyzed by spreading on the fourth day after transfection with SCM2 siRNA. Cells were treated with demecoline for four hours and mitotic cells were collected by shake-off. Chromosome spreads were prepared as described in Methods. D) Disorganization of metaphase alignment in condensin-depleted cells. Loss of visual separation between sister centromeres (CREST staining) and their delocalization from the spindle mid-zone after SMC2 depletion. Only partial Z-section projections are shown in CREST/tubulin panels (marked with arrows) to better illustrate the loss of ordered metaphase alignment. E) SMC2 depletion causes elongated spindles and reduced inter-polar tension. HeLa cells were treated as in (A) and stained with anti-cyclin B (cell cycle marker), anti-γ-tubulin (spindle pole marker) and CREST antibodies. DNA is stained with Hoechst 33342. Deconvolved images were used for spindle length measurements (shown with arrows) and spindle midzone positioning (solid line at CREST panel) using the anti-γ-tubulin label. Dashed lines denote the dispersion of centromeres in condensins-depleted cells versus control. F) SMC2 depletion produces elongated and deformed centromeres. HeLa cells treated as in (E) are stained with α-tubulin antibodies (spindle marker), CREST antibodies and Hoechst 33342. The distances between the outer edges of sister centromeres were measured in deconvolved (as shown) images. G,H) Ectopic expression of RNAi-resistant SMC2-2 restores SMC-2 levels, chromosome alignment, and centromere stretching. G) Immunoblotting for endogenous SMC2 or ectopic SMC2-2 mutant protein detected four days after siRNA transfection and two days after SMC2-2 transfection. CDC2 – loading control. H) HeLa cells transfected with SMC2 siRNA or mismatched siRNA (control RNAi) were retransfected 48 h later with a mixture of SMC2-2- and GFP-expressing plasmids. More than 95% of GFP-positive metaphases had wild-type metaphase plate morphology (bottom panels), while GFP-negative cells (upper panels) showed characteristic condensin-depletion defects.