Table 1.
Optimization Studies
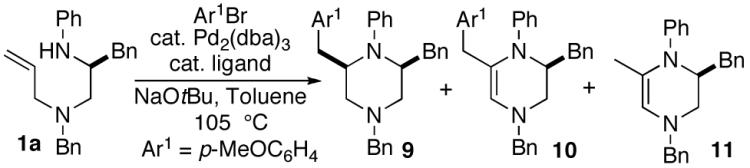 | ||||
|---|---|---|---|---|
| entry | ligandb | conversion (%)c | product ratiod | isolated yield |
| 1 | dppee | 50 | 68:0:32 | — |
| 2 | Dpe-Phos | 97 | 56:23:21 | — |
| 3 | Xantphos | 82 | 50:35:15 | — |
| 4 | P(o-tol)3 | 87 | 9:17:74 | — |
| 5 | P(2-furyl)3 | 97 | 71:7:21 | 62%e |
Conditions: 1.0 equiv 1a, 1.2 equiv Ar1Br, 1.2 equiv NaOtBu, 1 mol % Pd2(dba)3, 4 mol % chelating ligand or 8 mol % monodentate ligand, toluene (0.2 M), 105 °C, 8 h.
Dppe = 1,2-bis(diphenylphosphino)ethane, Dpe-Phos = 1,1-bis(diphenylphosphinophenyl)ether, Xantphos = 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene.
Conversion refers to % starting material consumed; 9-11 were the sole products detected in the crude reaction mixture.
Determined by 1H NMR analysis of crude reaction mixtures.
This yield was obtained after complete conversion.
