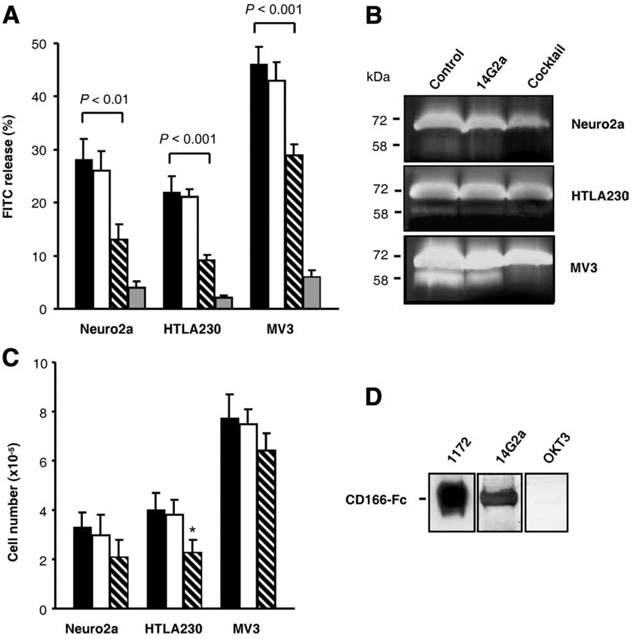
FIGURE 4.
Interaction of 14G2a mAb with recombinant and cellular CD166 glycoprotein. A, 14G2a mAb inhibits collagenolysis. Migration-associated collagenolysis caused by Neuro2a, HTLA230, and MV3 cells within three-dimensional FITC-collagen lattices was quantified from the FITC release after 72 h of migration in the presence or absence of inhibitors. Black, white, hatched, and gray bars denote cells cultured with medium, OKT3 mAb, 14G2a mAb, and the protease inhibitor cocktail. B, Inhibition of MMP-2 activation by 14G2a mAb. Neuro2a, HTLA230, and MV3 cell lines were cultured in collagen gels for 72 h. Conditioned media were analyzed by gelatin zymography to determine the level of MMP-2 activation. Position of 72 kDa pro-MMP-2 and 58 kDa active MMP-2 is indicated. C, Effect of 14G2a mAb on cell growth. Neuro2a, HTLA230, and MV3 cells were treated with 14G2a mAb (30 µg/ml) for 72 h and then counted with the trypan blue exclusion method. All determinants were made in triplicate samples, and the results are presented as the means ± SD of two independent experiments. *, p < 0.05. D, Western blotting analyses of recombinant CD166-Fc fusion protein (2 µg/lane) with mAbs specific for CD166 (1172), GD2 (14G2a), and CD3 (OKT3).