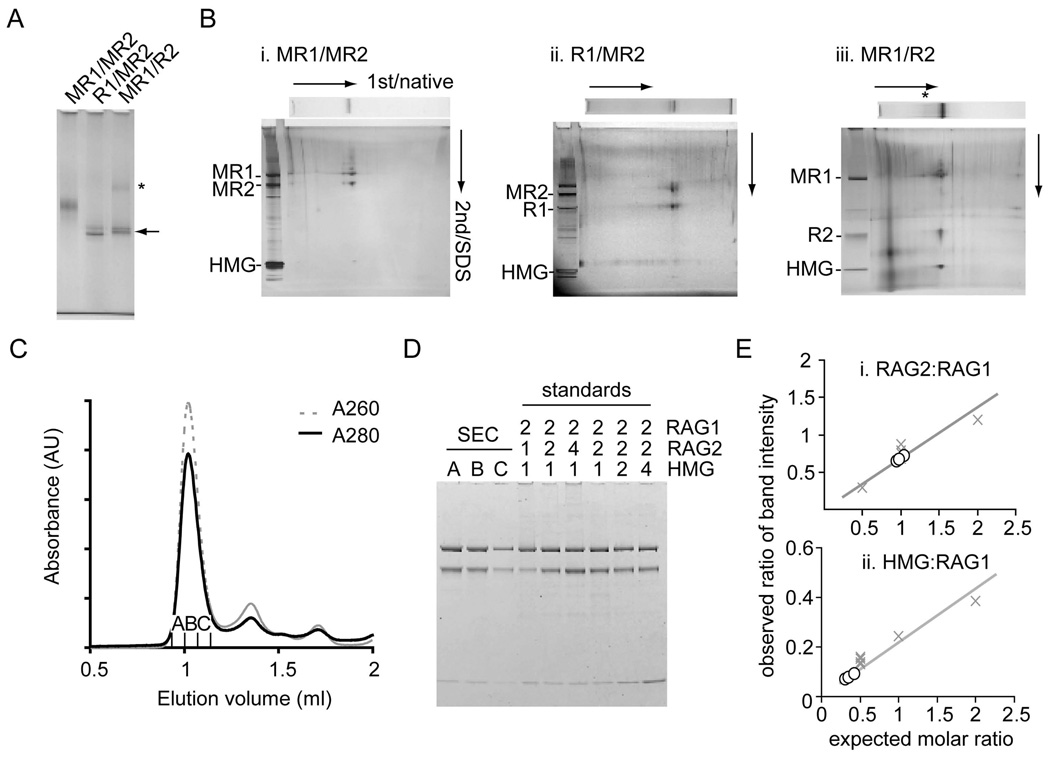
Figure 2. Protein components and stoichiometry of SEC.
(A) MBP was removed from RAG1 (R1/MR2) or RAG2 (MR1/R2) of the purified SEC using PreScission protease. The mobilities of digested SEC were compared on a 3–8% Tris-acetate native gel (arrow). The SEC was less soluble when MBP was removed from RAG2 and a SEC dimer may be evident (*).
(B) Protein components of SEC and PreScission-digested SEC from a native gel (7% or 3–8%) were separated in the second dimension by SDS-PAGE. Silver staining confirmed the presence of RAG1, RAG2 and HMGB1 as components of each purified complex. Analysis by 2D gel electrophoresis confirmed complete removal of MBP from AR1 or LR2 fusions, referred to as R1 and R2.
(C) SEC made from MR1 and LR2 was purified by gel-filtration chromatography (Superdex 200) using binding buffer adjusted to 150 mM KCl as running buffer.
(D) Three fractions from the major peak were loaded onto an SDS-PAGE gel alongside standards of known molar stoichiometries (RAG1:RAG2:HMGB1). The gel was stained with Coomassie Blue and the intensity of the bands in each lane was measured with a fluorescence scanner.
(E). Relative ratios of band intensity of RAG2:RAG1 and HMGB1:RAG1 were calculated for each fraction (open circle) and compared to the stoichiometry standards (X). There are 2 RAG2s and ≤1 HMGB1 per RAG1 dimer.