Abstract
The current study assessed the effects of developmental PCB and/or MeHg exposure on an operant task of timing and inhibitory control and determined if amphetamine (AMPH) drug challenges differentially affected performance. Long-Evans rats were exposed to corn oil (control), PCBs alone (1 or 3 mg/kg), MeHg alone (1.5 or 4.5 ppm), the low combination (1 mg/kg PCBs + 1.5 ppm MeHg), or the high combination (3 mg/kg PCBs + 4.5 ppm MeHg) throughout gestation and lactation. An environmentally relevant, formulated PCB mixture was used. Male and female offspring were trained to asymptotic performance on a differential reinforcement of low rates (DRL) operant task as adults. PCB-exposed groups had a lower ratio of reinforced to non-reinforced responses than controls. Groups exposed to MeHg alone were not impaired and the deficits observed in PCB-exposed groups were not seen when PCBs were co-administered with MeHg. AMPH was less disruptive to responding in males receiving PCBs alone, MeHg alone, and 1.0 mg/kg PCB + 1.5 ppm MeHg. Paradoxically, the disruption in responding by AMPH in males given 3.0 mg/kg PCB + 4.5 ppm MeHg did not differ from controls. Exposed females from all treatment groups did not differ from controls in their AMPH response. Overall, the findings suggest that developmental exposure to PCBs can decrease DRL performance. Co-exposure to MeHg seemed to mitigate the detrimental effects of PCBs on performance. The finding that the disruptive effects of AMPH on DRL performance were lessened in some groups of exposed males suggests that alterations in dopaminergic functioning may have a role in behavioral changes seen after perinatal PCB and MeHg exposure.
Keywords: PCBs, MeHg, amphetamine, developmental toxicity, impulsivity, inhibition
1. Introduction
Over the past several years, the public health importance of understanding the effects of co-exposure to different classes of environmental contaminants has come to the forefront. Multiple contaminants are found in the same exposure sources, and co-exposure may result in additive or synergistic neurotoxic effects. Polychlorinated biphenyls (PCBs) and methylmercury (MeHg) are two well known neurotoxicants that accumulate in fish (Easton et al. 2002; Weihe et al. 1996). The impact of prenatal exposure to PCBs and/or MeHg on neurodevelopment has been assessed in several prospective birth cohorts (for reviews see Grandjean 2006and Schantz et al. 2003). Both chemicals have been associated with poorer performance on cognitive and behavioral tests, although not all studies have reported exposure-related deficits (e.g. Gladen and Rogan 1991; Davidson et al. 2006). In addition, some studies have suggested possible interactions between the two chemicals (e.g. Grandjean et al. 2001; Stewart et al. 2003). Interestingly, a number of studies examining the effects of developmental exposure to PCBs or MeHg in humans or in animal models have reported deficits on tasks that require inhibitory control for their successful execution. The neurochemical basis for the inhibitory control deficits also appears to be similar for these two xenobiotics.
Developmental exposure to PCBs has been shown to produce an increase in perseverative-type responding (indicative of an inhibitory control impairment) in rats (Widholm et al. 2001; Widholm et al. 2004), monkeys (Levin et al. 1988; Rice and Hayward 1997), and prenatally exposed children (Jacobson and Jacobson 2003). During fixed-interval (FI) operant tasks, where only the response at the end of a pre-specified time interval is rewarded, gestational and lactational exposure of rats (Holene et al. 1998; Lilienthal et al. 1990) and monkeys (Mele et al. 1986; Rice 1997) to PCBs has been shown to result in increased responding relative to non-exposed controls. Treated animals generally respond more frequently during the interval (especially the early part) and have a shorter average inter-response time (IRT) resulting in a much higher number of responses emitted per reinforcer earned.
A similar type of response pattern (shorter IRTs and more responses made per reinforcer earned) has been seen subsequent to developmental PCB exposure in rats (Holene et al. 1999), monkeys (Rice 1998), and children (Stewart et al. 2006) during differential reinforcement of low rates of response (DRL) operant tasks. DRL tasks measure timing and inhibitory control and differ from FI tasks in that the interval clock resets if a response is made before the end of the interval. Developmental PCB exposure has also been associated with an increase in responding during extinction after FI or DRL testing (Holene et al. 1998; Sable et al. 2006). As extinction responding is never reinforced, a failure to withhold responding during extinction is also representative of an inhibitory control deficit.
Not all studies report negative effects of developmental PCB exposure on FI (Holene et al. 1999; Rice and Hayward 1998; Taylor et al. 2002), DRL (Rice and Hayward 1998; Sable et al. 2006), or extinction (Holene et al. 1999) tasks. These divergent results underscore the potential influence of factors such as dose, timing of exposure, sex of the animal and chlorine substitution pattern of the PCB congener or mixture on task performance. For example, male (Holene et al. 1998) but not female (Holene et al. 1999) rats exposed to the same ortho-substituted PCB congener responded excessively on FI and extinction tasks. In contrast, rats exposed perinatally to a coplanar PCB congener were not impaired on FI (Rice and Hayward 1998), which is consistent with the literature demonstrating that developmental exposure to coplanar PCBs generally have few effects on neurobehavioral function (Sable and Schantz 2006).
Developmental MeHg exposure has been shown to increase perseverative responding in rats performing spatial and fixed or progressive ratio tasks (Paletz et al. 2006; Reed et al. 2006; Widholm et al. 2004), to impair FI performance in rats (Reed and Newland 2007) and monkeys (Rice 1992), and to impair DRL performance in rats (Paletz et al. 2006) and children (Stewart et al. 2006). However, some investigators report either no or very limited impairment on FI responding in monkeys (Gilbert et al. 1996) and DRL performance in rats (Eccles and Annau 1982). The differences in results across studies can potentially be attributed to differences in methodology. The monkeys tested by Rice (1992) were given both in utero and direct postnatal exposure to MeHg and tested as infants or juveniles, while the monkeys tested by Gilbert et al. (1996) received only in utero exposure and were tested as adults. Infant blood mercury levels at birth were similar in the two studies, suggesting the importance of the postnatal exposure period in producing the FI impairment seen in the study by Rice (1992). Likewise, the differences in the DRL results of Eccles and Annau (1982) and Paletz et al. (2006) are likely due to the different exposure paradigms in these two studies. Rats were administered a single MeHg dose on gestational day 8 or 15 in the former study, while rats were exposed throughout gestation and via lactation until postnatal day (PND) 16 in the latter study.
Dopamine in the prefrontal cortex (PFC) has been shown to be critical for tasks of executive function including inhibitory control (Chudasama and Robbins 2006; Dalley et al. 2004; de Bruin et al. 2000; Robbins 2000; van der Meulen et al. 2007), and both PCB (Fonnum et al. 2006; Goodwill et al. 2007; Lyng et al. 2007) and MeHg (Faro et al. 2007; Kalisch and Racz 1996; McKay et al. 1986; Minnema et al. 1989) exposure have been reported to decrease brain dopamine (DA) levels in rats. These findings provide a potential neurochemical mechanism for the previously described behavioral results and also provide more evidence for the likelihood that PCBs and MeHg may have interactive effects. Some in vitro studies have suggested that MeHg exposure may exacerbate the reductions in DA produced by PCBs (Bemis and Seegal 1999), although not all researchers have found this effect (Castoldi et al. 2006).
Interestingly, the inhibitory control impairments associated with developmental PCB or MeHg exposure have remarkable similarities to the impulsivity that is known to be a core clinical symptom of ADHD. Recent discussions of animal (Carpenter et al. 2002; Holene et al. 1998; Rice 2000; Sagvolden et al. 2005; Widholm et al. 2004) and epidemiological (Grandjean and Landrigan 2006; Weiss and Landrigan 2000) studies of PCB exposure have highlighted this similarity. A reduction in PFC DA activity is believed to underlie the deficits in inhibitory control in ADHD children (Sullivan and Brake 2003) and pharmacotherapies that increase DA release are commonly used for treatment (Arnsten 2006).
To examine the nature of this similarity more closely, rats exposed to PCBs and/or MeHg perinatally were tested on a DRL task and subsequently a series of amphetamine (AMPH) drug challenges were conducted to determine if a drug that increases DA release would differentially alter performance in exposed and control animals. The PCB congener mixture and the ratio of PCBs to MeHg mimicked the contaminant profile found in fish in the Fox River in northeastern Wisconsin (Kostyniak et al. 2005). Consumption of fish from the Fox River is an ongoing source of PCB and MeHg exposure for humans. It was hypothesized that exposure to either contaminant would result in impaired performance on the DRL task, and that co-exposure would produce greater deficits than either contaminant alone. In addition, given the effects of these contaminants on the DA system, a shift in the dose response curve for AMPH was expected such that the performance of exposed animals would be less disrupted by AMPH than that of controls.
2. Methods
2.1. Animals
Ninety-two nulliparous female Long-Evans rats, approximately 60 days of age, were purchased in three cohorts spaced approximately 6 months apart from Harlan (Madison, WI). Animals used in these procedures were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were individually housed in standard plastic shoebox cages with corn-cob bedding, in a temperature- and humidity-controlled room (22°C, 40–55% humidity) on a 12-hour reverse light-dark cycle (lights off at 0830h). Standard rat chow and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health 2002) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
2.2. Exposure
After two weeks of adaptation to the vivarium, rats were assigned to exposure groups (balanced for body weight) and given one of seven treatments consisting of PCBs and/or MeHg (see Table 1). Exposure began 28 days prior to breeding in order to establish steady state PCB and MeHg concentrations and then continued until pups were weaned on PND 21. The PCB mixture was formulated to mimic the congener profile found in walleye, a popular fish for sports anglers, taken from the Fox River in northeast Wisconsin. The mixture consisted of 35% Aroclor 1242 (Monsanto Lot KB 05-415), 35% Aroclor 1248 (AccuStandards Lot F-110), 15% Aroclor 1254 (Monsanto Lot KB 05-612), and 15% Aroclor 1260 (AccuStandards Lot 021-020). The mixture was found to have relatively low aryl hydrocarbon receptor (AhR) activity, but high ryanodine receptor (RyR) activity (see Kostyniak et al. 2005). The doses of the PCB mixture (1 and 3 mg/kg) were selected based on the results of an earlier study assessing the in vivo developmental toxicity of the mixture in rats (see Kostyniak et al. 2005). The PCB mixture was diluted in corn oil (Mazola®) and pipetted onto one-half of a vanilla wafer cookie (Keebler Golden Vanilla Wafers®) at a volume of 0.4 mL/kg. The PCB-contaminated cookies were fed to the female rats daily at approximately 1100 hr. Doses were adjusted daily to account for weight gain. Corn oil vehicle alone was pipetted onto cookies for rats in treatment groups that did not receive PCBs. PCB dosing continued until the pups were weaned on PND 21. Methylmercury II chloride (Alfa Aesar; Wood Hill, MA) was administered in tap drinking water at concentrations of 1.5 and 4.5 ppm when rats were in their home cages. Unadulterated drinking water was given to rats in treatment groups that did not receive MeHg and to all male rats used for breeding. Due to concern about the pups being able to reach the water bottle, MeHg dosing of the dams stopped on PND 16. The doses for the combined exposure groups were selected to yield a ratio of PCBs to MeHg similar to that measured in walleye from the Fox River. The average daily amount of MeHg consumed across the entire dosing period was measured for dams in the first cohort only (see Table 1) and resulted in a ratio of PCBs to MeHg that was approximately 4.4:1 and 4.9:1 in the low and high combined exposure groups, respectively.
TABLE 1.
MeHg Intake and Reproductive Success for Each of the Treatment Groups
| Treatment Group1 | MeHg Intake2 (μg/kg) | Litters of ≥ 8 Pups (n) | Litters of ≤ 7 Pups4 (n) | Non-Pregnant Females (n) |
|---|---|---|---|---|
| Control | N/A | 9 | 2 | 6 |
| 1 mg/kg PCB | N/A | 8 | 2 | 2 |
| 3 mg/kg PCB | N/A | 8 | 2 | 1 |
| 1.5 ppm MeHg | 209.6 ± 18.4 | 8 | 1 | 3 |
| 4.5 ppm MeHg | 715.7 ± 58.2 | 9 | 2 | 1 |
| 1 mg/kg PCB/1.5 ppm MeHg | 228.8 ± 22.0 | 10 | 2 | 4 |
| 3 mg/kg PCB/4.5 ppm MeHg | 611.9 ± 55.13 | 9 | 0 | 3 |
PCBs dissolved in corn oil.
MeHg dissolved in drinking water reported as average daily intake (mean ± SEM) for the entire exposure period for cohort 1.
Significant difference from 4.5 ppm MeHg group (p=0.031).
Pups from these litters were not included in the study.
2.3. Breeding, pregnancy, and weaning
Four weeks after the initiation of exposure to PCBs and/or MeHg, each female was paired with an unexposed male Long-Evans rat (Harlan; Madison, WI) in a hanging wire cage for eight consecutive days. The breeding cages contained standard rat chow and standard tap water (ad libitum) to ensure that the males did not receive any MeHg exposure. The females were returned to their home cages each day for PCB dosing, where access to MeHg water was also available to MeHg exposure groups. During the breeding period, rats in both PCB/MeHg combined groups drank 18 to 31% less MeHg adulterated water on average than during the pre-breeding period, while this difference was not as noticeable for the MeHg only groups, which drank 5% less to 8% more MeHg water on average during the breeding period. The reason for these differences between groups in MeHg water consumption is unclear. There was no difference in total MeHg intake between the 1.5 ppm MeHg alone and 1.0 mg/kg PCB + 1.5 ppm MeHg groups. However, there was a significantly lower total MeHg intake in the 3.0 mg/kg PCBs + 4.5 ppm MeHg group relative to the 4.5 ppm MeHg alone group (Table 1). Consumption of the PCB adulterated cookie was confirmed before the females were returned to the breeding cages. All females consumed their cookie and were returned to their breeding cages within 30 min. The females were monitored twice daily for the presence of a sperm plug in order to establish gestational day (GD) 0, but were paired with the male for the entire 8 days of breeding. Females that did not give birth were retained and their uteri examined for implantation sites.
On the day of parturition (PND 0), the pups were examined for gross abnormalities, sexed and weighed, and the number of stillbirths noted. On PND 2, the litters were culled to 10 pups (5 males and 5 females when possible). Cross-fostering with extra pups from the same treatment group was done where possible to increase the size of small litters and assure all litters had 8–10 pups. However, in some cases pups of the same age from the same treatment group were not available for fostering. Litters consisting of ≤ 7 pups that could not be increased by fostering were not included in the study. Cross-fostered pups were marked by ear clip and were not used for behavioral testing. Thereafter, pups were weighed weekly until approximately PND 70. There were 61 successful litters. Of the remaining females, 20 were not pregnant and 11 had litters that were too small (i.e., ≤ 7 pups) to be included in the study (see Table 1). Overall, the non-pregnant females and small litters were relatively evenly distributed across treatment groups.
On the day of weaning, the dam from each litter was euthanized and her liver weight and the number of uterine implantation sites were recorded. Two males and two females from each litter were retained - one male and female for cognitive testing (reported here) and one male and female for auditory testing (data to be reported separately). The remaining pups in each litter were euthanized and organ weights (brain, liver, thymus) were obtained from one male and female per litter (when available).
Pups not euthanized on the day of weaning were housed in same-exposure, same-sex pairs or triplets. Beginning at PND 90 to 99, food access was restricted to reduce body weights to 250 ± 10 g for female rats and 350 ± 10 g for male rats. Food was restricted so that food rewards could be used as motivation to learn the behavioral task. Food restriction has been used routinely in our lab to study the behavioral effects of PCB exposure, and there is no evidence that it confounds PCB-mediated effects.
2.4. Apparatus
Behavioral testing was conducted in 16 automated operant chambers (Med-Associates; St. Albans, VT) housed in sound-attenuated wooden boxes, each ventilated by a fan (see Widholm et al. 2001). All operant chambers contained two retractable response levers and two stimulus cue lamps located symmetrically on both sides of the pellet trough. A white-noise generator masked extraneous sounds, and a sonalert speaker was used to signal reinforcement. The experimental contingencies were programmed using Med-State behavioral programming language (Med-Associates; St. Albans, VT).
2.5. Procedure
2.5.1. Autoshaping
Operant testing occurred six days per week. Beginning at PND 104 to 113, all animals were shaped to press the response levers by using an autoshaping program (see Newland et al. 1986; Widholm et al. 2001; Widholm et al. 2003). Reinforcement consisted of a single 45-mg food pellet (Purina TestDiet® grain-based formula; Richmond, IN) and the presentation of a 40-ms tone. Autoshaping sessions terminated after 60 min had elapsed or 100 reinforcers were delivered, whichever occurred first. A performance criterion of 100 lever presses within a single session was established, which rats met in a median of 2 sessions.
2.5.2. Lever press training
Following autoshaping, all animals were exposed to a continuous reinforcement schedule (see Widholm et al. 2001; Widholm et al. 2003) in which the cue-light and lever that were reinforced were alternated following the delivery of every fifth reinforcer. The purpose of this schedule was to strengthen the recently acquired lever press response and to prevent the rats from developing a lever or side preference prior to the start of cognitive testing. Each lever press training session terminated after either 100 reinforcers were delivered or 60 minutes had elapsed. A performance criterion of 100 reinforcers for at least two consecutive sessions was required to complete lever press training. The rats completed the lever-press training in a median of 3 sessions.
2.5.3. DRL training
Prior to DRL testing, all rats were tested on spatial reversal-learning (RL), delayed spatial alternation (DSA), and differential reinforcement of high rate (DRH) tasks (data to be reported elsewhere). Immediately following DRH, rats were trained on the DRL task, which began at approximately PND 180. Only the right lever was presented during all phases of the DRL task. During the first phase, a 1 s inter-response time (IRT) (DRL 1) was required in order to obtain a reinforcer. The first training phase lasted for two sessions regardless of performance. During the second and third phases, the IRT required for reinforcement was increased to 5 s (DRL 5) for 2 sessions and then 10 s (DRL 10) for 2 sessions. During each training phase animals were rewarded for the first lever press response occurring after the specified time interval had elapsed. Responses occurring before the required IRT had elapsed reset the timer, requiring the animal to wait another full interval before a response would result in reinforcement. All training sessions terminated after 200 reinforcers were delivered or 90 min had elapsed, whichever occurred first. Following DRL training, rats were given 30 daily sessions that required a 15 s IRT in order to obtain a reinforcer (DRL 15). Similar to the training phases, responses during the 15 s interval reset the timer and delayed reinforcement. Daily sessions terminated after 200 reinforcers were delivered or 90 min had elapsed, whichever occurred first.
2.5.4. Amphetamine drug challenge
After 30 days of testing on the DRL 15 task, AMPH drug challenges were implemented. d-Amphetamine sulfate (Sigma; St. Louis, MO) was prepared daily by dissolving it in 0.9% sterile saline to concentrations of 0.5 and 1.0 mg/mL for the 0.5 and 1.0 mg/kg doses, respectively. Dosages were selected based on their previous use in similar behavioral experiments (Ferguson et al. 2001; Wiley et al. 2000). All doses were mixed and maintained in areas of low light to prevent photodecomposition.
Drug challenge testing was identical to the previous DRL 15 sessions except that on Tuesdays and Fridays, each rat was given an injection of 0, 0.5 or 1.0 mg/kg AMPH intraperitoneally 10 minutes before DRL testing began. Two replicates of each AMPH dose were given to each rat in a counterbalanced Latin square design.
2.6. Data analysis
All statistical analyses were conducted using SPSS for MS Windows (version 15.0, SPSS Inc.; Chicago, IL) with statistical significance set at p<0.05. In the case of some repeated measures factors, a sphericity violation was noted. In such cases, a Greenhouse-Geisser correction was used to reduce the risk of a Type I error if ε was < 0.75 and a Huynh-Feldt correction was used when ε was > 0.75 (Rogan et al. 1979). Analyses requiring such correction are reported in the results using the appropriate adjusted degrees of freedom. When significant treatment effects were obtained from the overall analyses, additional post hoc simple effects and LSD analyses were conducted. Post hoc LSD analyses were done to allow comparison between exposure groups and the control group as well as comparisons between low and high dose exposure groups and between single and combined exposure groups. In the interest of brevity, only significant treatment- or AMPH-related effects are presented.
2.6.1. Reproductive/developmental endpoints
Reproductive data analyzed included litter size, percent male births, percent live births, percent gestational weight gain, and percent lactational weight gain. Percent gestational weight gain was determined by calculating (GD 21 weight - conception weight) / conception weight. Percent lactational weight gain was determined by calculating (highest lactational weight - weight after parturition) / weight after parturition. The ratio of liver:body weight and number of uterine implantation sites in the dam at weaning were also measured. For each dependent variable, a 7 (treatment) × 3 (cohort) between-subjects ANOVA was conducted.
Developmental data analyzed included postnatal weight gain and organ:body weight ratios. Day of eye opening was analyzed but no significant differences between groups were found. Postnatal weight gain was determined by collecting body weights on PND 0, 7, 14, and 21. These data were analyzed via a 7 (treatment) × 3 (cohort) × 2 (sex) × 4 (age) mixed ANOVA with sex (nested within litter) and age as repeated measures factors. Organ to body weight ratios for brain, liver, and thymus were measured on the day of weaning and analyzed separately via a 7 (treatment) × 3 (cohort) × 2 (sex) mixed ANOVA with sex nested within litter.
2.6.2. DRL dependent measures
Data from DRL 1, DRL 5, and DRL 10 were analyzed separately using 7 (treatment) × 3 (cohort) × 2 (sex) × 2 (day) mixed ANOVAs. For DRL 15, data were averaged across five day blocks to yield 6 testing blocks. The two primary measures of learning were the ratio of reinforced:non-reinforced responses and number of reinforcers delivered. These dependent measures were analyzed separately using a 7 (treatment) × 3 (cohort) × 2 (sex) × 6 (block) mixed ANOVA where sex (nested within litter) and testing block were repeated measures factors. Each response made during DRL 15 was also cataloged into one of eight 2.5 s IRT bins. The proportion of responses falling within each IRT bin was calculated and averaged across the 5 days within the first testing block (acquisition) and sixth testing block (steady state). Each of these was analyzed separately via a 7 (treatment) × 3 (cohort) × 2 (sex) × 8 (bin) mixed ANOVA where sex (nested within litter) and IRT bin were repeated measures factors.
2.6.3. Amphetamine Drug Challenge Dependent Measures
For the AMPH drug challenge, baseline values were obtained by averaging the ratio of reinforced:non-reinforced responses and the number of reinforcers delivered across all days when injections were not given during the 3 weeks of drug challenge testing. The same dependent variables were obtained for each of the AMPH doses by averaging across the two replications for each dose. To control for the fact that there were treatment differences in DRL performance prior to the drug challenge phase, a difference score was calculated for each dose relative to baseline and the dependent variables were expressed as a percent change from baseline. This allowed for examination of the effects of AMPH while controlling for previous differences in DRL performance among the treatment groups. These two dependent variables were analyzed separately using a 7 (treatment) × 3 (cohort), × 2 (sex) × 3 (AMPH dose) mixed ANOVA where sex (nested within litter) and AMPH dose were repeated measures factors.
3. Results
3.1. Reproductive/developmental endpoints
The dams did not exhibit overt clinical signs of toxicity in any of the treatment groups. A summary of all reproductive outcomes can be found in Table 2, so only key significant differences will be summarized here. There was a significant treatment × cohort interaction [F(12,40)=2.609, p=0.011] on percent gestational weight gain. Within cohort 1 only, dams in the 1 mg/kg PCB group (p=0.021), 3 mg/kg PCB group (p=0.005), and 3.0 mg/kg PCB + 4.5 ppm MeHg group (p=0.017) gained significantly less weight than control dams (p=0.019). There were no other significant differences in gestational weight gain between the control animals and the treated animals in cohort 1, 2, or 3. There was also a near significant treatment × cohort interaction [F(12,40)=1.985, p=0.052] for percent lactational weight gain. However, simple effects analyses for treatment within each cohort did not reveal any significant differences.
TABLE 2.
Reproductive Outcomes of Dams Exposed to the Fox River PCB Mixture ± MeHg during Gestation and Lactation1
| Exposure Group PCB / MeHg | % Gestational Weight Gain (% pre-pregnancy) | % Lactational Weight Gain (% change from day of parturition) | Dam Liver Weight | Dam Liver Weight:Body Weight | Litter Size | % Male | % Live Births | Implantation Sites |
|---|---|---|---|---|---|---|---|---|
| 0 mg / 0 ppm (Control; n=9) | 49.81 ± 3.56 | 10.64 ± 1.96 | 12.88 ± 0.49 | 0.0464 ± 0.0012A | 10.56 ± 1.19 | 47.66 ± 4.84 | 100.00 ± 0.00 | 10.67 ± 0.96 |
| 1 mg / 0 ppm (n=8) | 53.02 ± 1.03 | 9.73 ± 1.92 | 13.16 ± 0.32 | 0.0462 ± 0.0004 | 12.13 ± 0.61 | 48.17 ± 2.56 | 100.00 ± 0.00 | 12.75 ± 0.65 |
| 3 mg / 0 ppm (n=8) | 47.17 ± 1.39 | 9.18 ± 1.43 | 14.41 ± 0.61 | 0.0481 ± 0.0007A,B | 10.75 ± 1.08 | 43.49 ± 4.58 | 100.00 ± 0.00 | 12.00 ± 1.00 |
| 0 mg / 1.5 ppm (n=8) | 46.53 ± 2.47 | 10.35 ± 2.04 | 12.52 ± 0.49 | 0.0438 ± 0.0009 | 9.75 ± 0.96 | 50.62 ± 4.02 | 99.07 ± 0.93 | 10.88 ± 1.08 |
| 0 mg / 4.5 ppm (n=9) | 49.50 ± 2.81 | 10.08 ± 1.24 | 12.94 ± 0.73 | 0.0445 ± 0.0011 | 10.11 ± 0.89 | 49.63 ± 2.79 | 100.00 ± 0.00 | 12.22 ± 0.83 |
| 1 mg / 1.5 ppm (n=10) | 51.22 ± 1.65 | 12.16 ± 0.98 | 13.74 ± 0.54 | 0.0476 ± 0.0009A,B | 11.50 ± 0.78 | 46.19 ± 4.40 | 100.00 ± 0.00 | 12.80 ± 0.83 |
| 3 mg / 4.5 ppm (n=9) | 48.67 ± 1.87 | 7.74 ± 1.53 | 13.44 ± 0.43 | 0.0472 ± 0.0005A,B | 11.67 ± 0.71 | 49.07 ± 5.01 | 100.00 ± 0.00 | 12.67 ± 0.55 |
Data calculated on litters included in behavioral study (≥7 pups); Means (± SEM); n = # of successful litters.
PCB = Polychlorinated biphenyls; MeHg = Methyl mercury
Significant difference from 1.5 ppm MeHg group (p<0.05)
Significant difference from 4.5 ppm MeHg group (p<0.05)
A main effect of treatment was revealed on the dam liver:BW ratio. Post hoc LSD analysis revealed that the 1.5 ppm MeHg group had a significantly lower liver:BW ratio than the control group (p=0.040), the 3.0 mg/kg PCB group (p=0.001), the 1.0 mg/kg PCB + 1.5 ppm MeHg group (p=0.003), and the 3.0 mg/kg PCB + 4.5 ppm MeHg group (p=0.009). There were no significant treatment effects related to litter size, percent males, percent live births, or implantation sites (Table 2).
Analyses of the data on postnatal weight gain (Table 3) revealed a significant main effect of treatment [F(6,40)=3.525, p=0.007] and a significant treatment × day interaction [F(7.035,46.901)=3.986, p=0.002]. Additional post hoc analyses revealed a significant effect of treatment only on PND 21. LSD analysis revealed a number of significant differences (indicated in Table 3) that were driven primarily by the higher body weights observed in the 1.5 ppm MeHg and 1.0 mg/kg PCB + 1.5 ppm MeHg groups relative to other exposure groups.
TABLE 3.
Body Weights and Organ Weight Ratios (g) for Pups Born to Dams Exposed PCBs ± MeHg during Gestation and Lactation1
| Exposure Group PCB/MeHg | Birth Weight (PND 0) | Weaning Weight (PND 21) | Brain:Body Weight Ratio | Liver:Body Weight Ratio | Thymus:Body Weight Ratio |
|---|---|---|---|---|---|
| 0 mg / 0 ppm (Control; 9 litters) | |||||
| MALE | 6.40 ± 0.11 | 46.02 ± 2.03BC | 0.0322 ± 0.0017 | 0.0370 ± 0.0007ABCD | 0.0042 ± 0.0002ABCD |
| FEMALE | 6.11 ± 0.10 | 43.39 ± 1.86BC | 0.0332 ± 0.0010 | 0.0374 ± 0.0008ABCD | 0.0049 ± 0.0004ABCD |
| 1 mg / 0 ppm (8 litters) | |||||
| MALE | 6.50 ± 0.19 | 48.10 ± 1.26 | 0.0312 ± 0.0011 | 0.0474 ± 0.0010BD | 0.0039 ± 0.0001 |
| FEMALE | 6.21 ± 0.18 | 46.54 ± 1.27 | 0.0325 ± 0.0012 | 0.0472 ± 0.0009BD | 0.0041 ± 0.0002 |
| 3 mg / 0 ppm (8 litters) | |||||
| MALE | 5.94 ± 0.18 | 43.36 ± 1.24ABC | 0.0335 ± 0.0010A | 0.0601 ± 0.0013C | 0.0037 ± 0.0001 |
| FEMALE | 5.91 ± 0.27 | 41.52 ± 1.02ABC | 0.0335 ± 0.0010A | 0.0604 ± 0.0008C | 0.0041 ± 0.0001 |
| 0 mg / 1.5 ppm (8 litters) | |||||
| MALE | 6.51 ± 0.11 | 49.63 ± 1.15 | 0.0302 ± 0.0009 | 0.0383 ± 0.0003ABCD | 0.0043 ± 0.0002D |
| FEMALE | 6.11 ± 0.14 | 48.11 ± 1.42 | 0.0321 ± 0.0014 | 0.0371 ± 0.0005ABCD | 0.0044 ± 0.0002D |
| 0 mg/ 4.5 ppm (9 litters) | |||||
| MALE | 6.64 ± 0.26 | 46.26 ± 2.74BC | 0.0334 ± 0.0017A | 0.0361 ± 0.0009ABCD | 0.0042 ± 0.0002ABCD |
| FEMALE | 6.39 ± 0.20 | 43.31 ± 1.96BC | 0.0335 ± 0.0015A | 0.0367 ± 0.0007ABCD | 0.0048 ± 0.0002ABCD |
| 1 mg/ 1.5 ppm (10 litters) | |||||
| MALE | 6.79 ± 0.14 | 50.24 ± 1.47 | 0.0301 ± 0.0009 | 0.0482 ± 0.0013 | 0.0040 ± 0.0002 |
| FEMALE | 6.43 ± 0.16 | 48.59 ± 1.46 | 0.0311 ± 0.0009 | 0.0484 ± 0.0022 | 0.0039 ± 0.0001 |
| 3 mg / 4.5 ppm (9 litters) | |||||
| MALE | 6.27 ± 0.10 | 44.58 ± 1.45BC | 0.0323 ± 0.0012 | 0.0609 ± 0.0011C | 0.0035 ± 0.0002 |
| FEMALE | 6.03 ± 0.07 | 43.24 ± 1.49BC | 0.0339 ± 0.0010 | 0.0632 ± 0.0013C | 0.0037 ± 0.0002 |
Data calculated on litters included in behavioral study (≥7 pups); Means (± SEM). g=grams; PCB =Polychlorinated biphenyls; MeHg = Methylmercury
Weaning Weight: Significant difference from 1.0 mg/kg PCB group
significant difference from 1.5 ppm MeHg group
significant difference from 1.0 mg/kg PCB + 1.5 ppm MeHg group (all p<0.05)
Brain:BW Ratio: Significant difference from 1.0 mg/kg PCB + 1.5 ppm MeHg group (p<0.05)
Liver:BW Ratio: Significant difference from 1.0 mg/kg PCB group
significant difference from 3.0 mg/kg PCB group, significant difference from
1.5 ppm PCB + 4.5 ppm MeHg group
Significant difference from 3.0 mg/kg PCB + 4.5 ppm MeHg group (all p<0.001)
Thymus:BW Ratio: Significant difference from 1.0 mg/kg PCB group
significant difference from 3.0 mg/kg PCB group, significant difference from
1.5 ppm PCB + 4.5 ppm MeHg group
significant difference from 3.0 mg/kg PCB + 4.5 ppm MeHg group (all p<0.05)
Analysis of the brain:BW ratio also revealed significant a main effect of treatment [F(6,40)=2.715, p=0.026], but none of the exposure groups were significantly different from controls. LSD analysis revealed that the brain:BW ratio in the 1.0 mg/kg PCB + 1.5 ppm MeHg group was significantly lower than the 3.0 mg/kg PCB group (p=0.032) and the 4.5 ppm MeHg group (p=0.031). Analysis of the liver:BW ratio also revealed a main effect of treatment [F(6,40)=115.580, p<0.001]. LSD analysis revealed that relative to controls and to the MeHg alone groups, a significantly higher liver:BW ratio was found in all exposure groups that received PCBs (p<0.001 for all comparisons). There was also a significant main effect of treatment [F(6,40)=4.755, p=0.001] on the thymus:BW ratio. LSD analysis revealed that relative to controls, a significantly lower thymus:BW ratio was found in all exposure groups that received PCBs (p≤0.05 in all cases). For all significant differences in organ weight:BW ratios, the differences were due to alterations in organ weights between groups and not due to changes in body weight.
3.2. DRL
3.2.1. DRL 1, DRL 5, DRL 10
For all of the training phases, there were no treatment related differences in the number of lever presses, reinforcers delivered, or the ratio of reinforced:non-reinforced responses with the exception of a significant exposure group × cohort × sex interaction [F(12,40)=2.043, p=0.045] on the number of lever presses during DRL 1. This effect occurred because some of the female treatment groups in cohorts 1 and 2 (different groups in the different cohorts) did not have 200 lever presses (with an associated 200 reinforcers) while most of the other treatment groups did. These female treatment groups did have an adequate average number of lever presses (range 169.5 – 198.3), and like the other animals, each lever press they made resulted in a reinforcer such that the ratio of reinforced:non-reinforced responses did not significantly differ across any of the treatment groups.
3.2.2. DRL 15
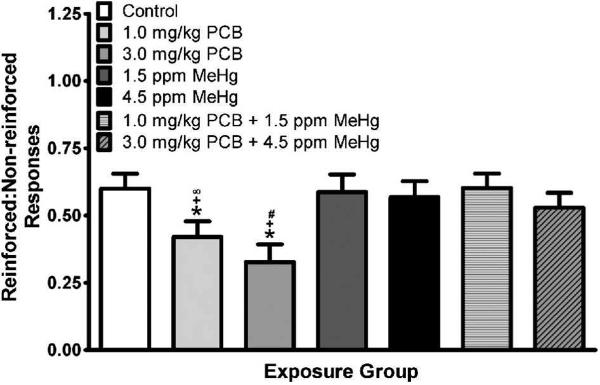
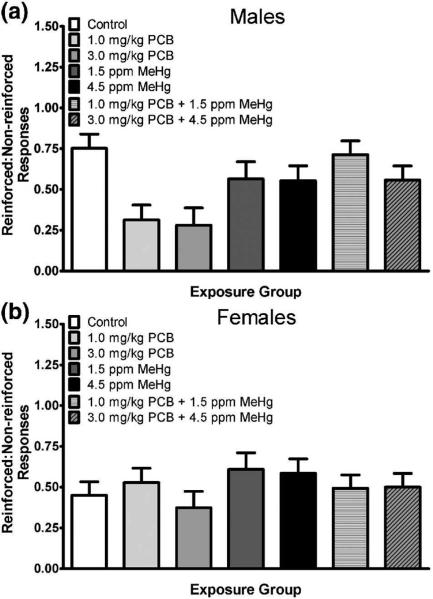
Analysis of the ratio of reinforced:non-reinforced trials revealed a significant main effect of treatment [F(6,40)=2.964, p=0.017] and block [F(3.185,127.382)=104.465, p<0.001], as well as a significant treatment × cohort interaction [F(12,40)=2.049, p=0.045]. Visual inspection of the data revealed that performance of the control rats was consistent across cohorts, whereas performance of PCB-exposed rats in the first cohort appeared to be somewhat less impaired by PCB exposure than that of PCB-exposed rats in the other two cohorts. The data representing the ratio of reinforced:non-reinforced trials across treatment groups are presented in Fig. 1. LSD post hoc analysis across these treatment marginal means indicated a significant difference between the control group and both the 1 mg/kg (p=0.033) and the 3 mg/kg (p=0.048) PCB groups. No other treatment groups were different from the control group. The 1 and 3 mg/kg PCB groups were also significantly different from the 1.5 ppm (p=0.023 and p=0.002, respectively) and 4.5 ppm MeHg (p=0.035 and p=0.004, respectively) alone groups as well as the 1.0 mg/kg PCB + 1.5 ppm MeHg group (p=0.015 and p=0.001, respectively). The difference between the 3.0 mg/kg PCB group and the 3.0 mg/kg PCB + 4.5 ppm MeHg was significant (p=0.006), while the difference between the 1.0 mg/kg PCB group and the 3.0 mg/kg PCB + 4.5 ppm MeHg group did not quite reach significance (p=0.053). Although the overall analysis did not reveal a significant treatment × sex [F(6,40)=1.799, p=0.124] or significant treatment × sex × block [F(16.079,107.191)=1.247, p=0.245] interaction, visual inspection of the data revealed that the treatment main effect was more profound in the males. Males given either dose of PCBs alone (but not when combined with MeHg) had a lower ratio of reinforced:non-reinforced trials indicating they did not perform as well on the task (see Fig. 2.). Follow up of the overall main effect of block revealed that the performance of the animals improved across blocks.
Fig. 1.
Ratio of reinforced:non-reinforced responses as a function of exposure group. Data are collapsed across cohort, sex, and testing block. *Significant difference from the control group (p<0.05), +Significant difference from the 1.5 ppm MeHg group and the 4.5 ppm MeHg group (p<0.05), ∞Significant difference from the 1.0 mg/kg PCB + 1.5 ppm MeHg group (p=0.15) with marginally significant difference from 3.0 mg/kg PCB + 4.5 ppm MeHg group (p=0.053), #Significant difference from the 1.0 mg/kg PCB + 1.5 ppm MeHg group and the 3.0 mg/kg PCB + 4.5 ppm MeHg group (p<0.01).
Fig. 2.
Ratio of reinforced:non-reinforced responses as a function of exposure group and sex. Data are collapsed across cohort and testing block. PCB-exposed males (panel a) appeared to be more impaired than PCB-exposed females (panel b).
Significant main effects of treatment [F(6,40)=2.388, p=0.046] and block [F(2.637,105.491)=226.814, p<0.001] were found for the number of reinforcers earned (data not shown). Once again, visual inspection of the data clearly revealed that males (but not females) given either dose of PCBs (but not when combined with MeHg) had a lower number of reinforced trials.
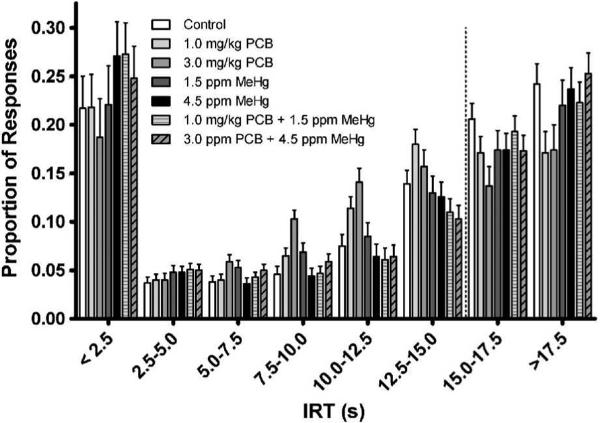
Analysis of the proportion of responses falling within each IRT bin during acquisition (block 1 only) revealed no significant treatment-related effects. The same analyses on steady-state data (block 6 only) revealed the treatment × IRT [F(14.083,93.889)=1.733, p=0.061] interaction approached significance. Animals given PCB exposure alone tended to make a higher proportion of responses with shorter IRTs (particularly 7.5 – 12.5 s) - responses that did not result in reinforcement. However, in the longer IRT bins where reinforcement would have occurred (i.e., responses with IRTs > 15 s), this pattern was reversed with PCB exposure resulting in a lower proportion of responses. This effect was not observed with MeHg exposure either alone or in combination with PCBs (Fig. 3.).
Fig. 3.
Proportion of responses falling within each inter-response time (IRT) bin during block 6 (steady-state performance). Only responses in the last two IRT bins resulted in the delivery of a reinforcer.
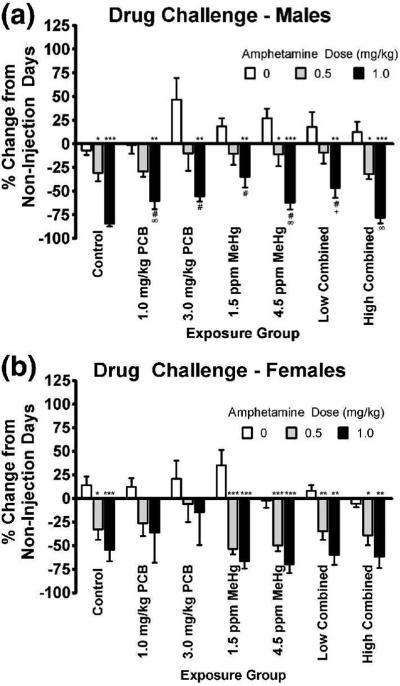
3.2.3. Amphetamine Drug Challenge
The total number of responses and reinforcers delivered as a function of treatment and AMPH dose are presented in Table 4. Analysis of the percent change (from baseline) in the ratio of reinforced:non-reinforced trials (Fig. 4) revealed significant main effects of treatment [F(6,40)=5.696, p<0.001] and AMPH [F(2,80)=139.016, p<0.001]. Significant interactions of treatment × cohort [F(12,40)=2.704, p=0.009], cohort × AMPH [F(4,80)=4.677, p=0.002], sex × AMPH [F(2,80)=5.595, p=0.005], and treatment × sex × AMPH [F(12,80)=2.877, p=0.002] were also found.
TABLE 4.
Effects of AMPH Drug Challenge on Total Responses and Reinforcers Delivered Following Developmental PCB and/or MeHg Exposure
| Males | Females | |||
|---|---|---|---|---|
| Total Responses | Reinforcers Delivered | Total Responses | Reinforcers Delivered | |
| Control | ||||
| 0 mg/kg AMPH | 318.36±33.70 | 147.67±11.28 | 316.22±35.48 | 109.06± 9.40 |
| 0.5 mg/kg AMPH | 385.97±41.35 | 144.03±8.33 | 416.89±48.40 | 89.17±9.99 |
| 1.0 mg/kg AMPH | 623.43±83.75 | 74.71±12.44 | 544.11±96.28 | 70.89±13.07 |
| 1 mg/kg PCB | ||||
| 0 mg/kg AMPH | 403.44±30.08 | 122.50±18.41 | 305.06±20.92 | 125.81±12.92 |
| 0.5 mg/kg AMPH | 446.50±42.78 | 106.63±13.44 | 353.50±34.39 | 101.75±10.30 |
| 1.0 mg/kg AMPH | 591.38±53.66 | 79.75±9.82 | 493.19±76.44 | 70.31±7.18 |
| 3 mg/kg PCB | ||||
| 0 mg/kg AMPH | 345.75±39.08 | 117.81±12.66 | 311.31±44.55 | 107.88±14.79 |
| 0.5 mg/kg AMPH | 440.31±34.86 | 106.94±19.19 | 375.00±52.12 | 103.31±13.50 |
| 1.0 mg/kg AMPH | 530.81±24.10 | 82.88±14.71 | 506.56±115.95 | 69.75±10.31 |
| 1.5 ppm MeHg | ||||
| 0 mg/kg AMPH | 328.38±24.50 | 134.50±11.64 | 320.56±39.03 | 153.13±13.03 |
| 0.5 mg/kg AMPH | 343.19±22.66 | 113.19±8.08 | 457.94±55.11 | 111.31±9.05 |
| 1.0 mg/kg AMPH | 405.69±30.26 | 103.50±5.96 | 552.50±81.73 | 89.88±8.95 |
| 4.5 ppm MeHg | ||||
| 0 mg/kg AMPH | 300.55±24.19 | 137.09±9.77 | 325.21±11.50 | 128.86±10.39 |
| 0.5 mg/kg AMPH | 373.64±39.49 | 127.03±7.05 | 412.86±15.17 | 102.64±7.67 |
| 1.0 mg/kg AMPH | 548.37±50.28 | 101.04±1 1.48 | 581.79±57.08 | 68.57±5.13 |
| 1 mg/kg PCB/1.5 ppm MeHg | ||||
| 0 mg/kg AMPH | 363.05±41.24 | 146.25±9.54 | 335.15±22.07 | 133.00±11.25 |
| 0.5 mg/kg AMPH | 338.75±24.61 | 137.50±9.86 | 406.60±27.28 | 110.75±10.15 |
| 1.0 mg/kg AMPH | 469.80±41.32 | 104.15±11.57 | 512.60±49.68 | 81.75±13.43 |
| 3 mg/kg PCB/4.5 ppm MeHg | ||||
| 0 mg/kg AMPH | 360.11±26.39 | 155.89±13.77 | 356.71±29.66 | 135.42±15.28 |
| 0.5 mg/kg AMPH | 410.61±37.84 | 133.61±14.62 | 410.10±35.09 | 111.30±9.95 |
| 1.0 mg/kg AMPH | 700.94±94.49 | 74.50±11.57 | 545.47±76.57 | 77.61±7.36 |
Note. Values reported as mean ± SEM.
PCB =Polychlorinated biphenyls; MeHg = Methylmercury; AMPH = Amphetamine
Fig. 4.
Percent change relative to non-injection days in the ratio of reinforced:non-reinforced responses as a function of amphetamine dose for the males (panel a) and females (panel b). Higher numbers represent an increase in performance relative to non-injection days. Low combined = 1.0 mg/kg PCB + 1.5 ppm MeHg, high combined = 3.0 mg/kg PCB + 4.5 ppm MeHg. Significant difference from 0 mg/kg amphetamine dose within the same exposure group at *p<0.05, **p<0.01, and ***p<0.001. Significant difference from #control group (p<0.05), ∞1.5 ppm MeHg group (p<0.05), and +high combined group (p<0.05) given 1.0 mg/kg amphetamine.
Follow up analyses comparing the effects of AMPH within each treatment group and within each sex (i.e., comparing the effects of AMPH within an individual male or individual female treatment group) were then conducted. Dunnett t-tests revealed that the 1.0 mg/kg dose of AMPH resulted in a significant decrease in performance in all male treatment groups relative to the 0 mg/kg AMPH dose, while the 0.5 mg/kg AMPH dose was not significantly different from the 0 mg/kg dose in some male treatment groups (see Fig. 4a). A similar trend was found in the females, with the exception that no significant effects of AMPH were found in the 1 mg/kg PCB or 3 mg/kg PCB groups (see Fig. 4b).
Follow up analyses of treatment effects within each level of AMPH for each sex (i.e., comparing the effects of treatment in males or females given the same dose of AMPH) were also conducted and revealed a significant effect of treatment following injection of 1.0 mg/kg AMPH in the male (Fig. 4a), but not the female (Fig. 4b) animals. Post hoc LSD analysis in the male animals receiving 1.0 mg/kg of AMPH revealed that all exposure groups, with the exception of the 3.0 mg/kg PCB + 4.5 ppm MeHg group (high combined), were significantly less impaired by the 1.0 mg/kg dose compared to control animals (p<0.05 for both PCB and MeHg groups, p=0.001 for 1.0 mg/kg PCB + 1.5 ppm MeHg group) (Fig. 4a). In addition, the 1.5 ppm MeHg group was significantly less impaired than the 1.0 mg/kg PCB group (p=0.035), the 4.5 ppm MeHg group (p=0.021), and the 3.0 mg/kg PCB + 4.5 ppm MeHg group (p<0.001) and the 1.0 mg/kg PCB + 1.5 ppm MeHg group was significantly less impaired than the 3.0 mg/kg PCB + 4.5 ppm MeHg group (p=0.005).
4. Discussion
Rats exposed to the Fox River PCB mix showed inhibitory control impairments demonstrated as a reduction in the ratio of reinforced to non-reinforced responses on the DRL 15 task relative to controls. These results are in agreement with the majority of the published literature examining the effects of developmental PCB exposure on inhibitory control in the DRL task (e.g., Holene et al. 1999; Rice 1998; Stewart et al. 2006). Visual inspection of the data revealed that the overall treatment effect seemed to be driven primarily by the males, who were more impaired by PCB exposure than the females. Control males performed better than control females and PCB exposed males appeared feminized in the sense that they performed similarly to control females (Fig 2). The performance of the female rats in this study was very similar to what we have observed in intact female rats tested on this same DRL task in previous studies (Wang et al. 2008). Aromatase, which converts testosterone to estradiol, has an important role in the sexual differentiation of the brain (Dickerson and Gore 2007; Roselli 2007) and aromatase levels in the brain are highest in the rat during the perinatal period, with males expressing higher levels than females (Lauber et al. 2007). Alterations in aromatase levels during early development influence DA concentrations in the prefrontal cortex (Stewart and Rajabi 1994). Prenatal exposure to a reconstituted PCB mixture designed to mimic the congener-pattern in human breast milk has been shown to reduce aromatase activity in the brain of newborn male rat pups (Hany et al. 1999). A separate study has also reported aromatase changes in rats exposed to both PCBs and MeHg. Perinatal exposure to fish diets containing PCB:MeHg ratios ranging from higher to lower than those employed in the current study reduced aromatase activity in the ovaries of adult rats (Gerstenberger et al. 2000). It is possible that the Fox River PCB Mix reduced aromatase activity such that estradiol production was decreased in our male pups. The resultant changes in brain differentiation and neurochemistry could explain why PCB exposed males performed similar to exposed and control females on the DRL task. Gender specific deficits have also been reported in perinatally PCB-exposed male rats tested on other cognitive tasks (Roegge et al. 2000; Widholm et al. 2001). In addition, Geller and colleagues reported that early exposure of rats to PCBs abolished the normal male-female difference in visual thresholds (Geller et al. 2001).
It should be noted that a few studies have reported no effects of PCBs on DRL tasks including a previous study published by our laboratory that measured DRL 15 performance following developmental PCB exposure (Rice and Hayward 1998; Sable et al. 2006). The discrepancy between the two studies conducted in our lab is difficult to explain as rats used in both studies were from the same commercial barrier facility, the same dosing solutions were used for an identical dosing duration, and animals were tested on the same behavioral test battery using the same equipment. In retrospect, we believe the inability to detect treatment related effects in the previous study may have occurred due to a relative lack of statistical power. Unlike the current experiment, we were not able to include cohort as a factor in the overall analysis in the previous study because we did not have successful litters in all of our treatment groups across cohorts. Thus, any variance associated with cohort effects contributed to the within-subjects error, thereby decreasing power and making it harder to detect treatment-related effects. It should also be noted that PCB-exposed rats from our previous study did show evidence of impaired inhibitory control, as measured by increased responding during extinction of the DRL task.
Developmental exposure to MeHg alone failed to impair DRL performance in the current study – a result that appears to be in contrast to previous studies reporting detrimental effects of developmental MeHg exposure on DRL performance in rats (Paletz et al. 2006) and humans (Stewart et al. 2006). There did not appear to be substantial differences in the MeHg exposures (as measured by adulterated water intake in the rats and hair MeHg in humans) that could account for these differences (Paletz et al. 2006; Stewart et al. 2006). There were, however, some notable differences in DRL testing among the studies. Rats in the current study were tested on a DRL 15s task for 30 sessions after being trained for 2 sessions each on shorter schedules of DRL 5s and DRL 10s (1 session/day). Rats in the Paletz et al. (2006) study were tested for only 4 sessions (1 session/day) on a DRL 10s task, and children in the Stewart et al. (2006) study were tested on a DRL 20s task in a single session lasting an hour at most. MeHg-exposed rats in the Paletz et al. (2006) study were impaired during the first session but were not impaired by the fourth session. Taken together, the results of all three studies suggest that MeHg exposure appears capable of producing deficits in DRL performance, but the effects may be transient or expressed only during learning.
The current study also found the surprising result that exposure to MeHg in combination with doses of PCBs that alone produced impairments did not result in deficits on the DRL task. In other words, the deficits observed in the PCB-exposed animals were not seen when the same PCB dose was administered in combination with MeHg. A similar interaction was found when littermates of the animals tested in the current study were tested for auditory deficits (Powers et al. in preparation). Specifically, a second male and female from each litter were tested for distortion product otoacoustic emissions (DPOAEs) as an assessment of cochlear function and auditory brainstem responses (ABRs) to determine effects on central auditory pathways. DPOAE amplitudes were decreased, and DPOAE and ABR thresholds were elevated across a range of frequencies in the PCB-exposed rats. However, when the same PCB doses were given in combination with MeHg, the decrease in DPOAE amplitude was absent and the increase in DPOAE and ABR thresholds were less pronounced.
Previous work in our laboratory examining the effects of developmental exposure to the commercial PCB mixture Aroclor 1254 in combination with MeHg indicated that MeHg exacerbated PCB related motor impairments on a rotating rod (Roegge et al. 2004), but did not exacerbate PCB-related impairments on a spatial alternation task (Widholm et al. 2004). However, in those studies a different PCB mixture, a higher PCB dose, and a lower MeHg dose were used resulting in a much higher ratio of PCBs to MeHg (102:1 during gestation). Overall, these results indicate PCBs and MeHg may interact in complex ways to produce functional changes in the brain, and the type and degree of interaction observed may be highly dependent on the PCB mixture, the ratio of PCBs to MeHg, and the type of endpoint that is assessed. While it is unlikely that the presence of MeHg would alter the tissue distribution of PCBs, at this time we cannot rule out the possibility that co-exposure to MeHg altered the delivery of PCBs to the brain. Evidence of the complexity of PCB/MeHg interactions has been shown in previous in vitro research where the dose and duration of exposure to these two contaminants influenced the neurochemical outcome. Co-exposure at lower concentrations of PCBs and MeHg resulted in synergistic effects on intracellular calcium release, while co-exposure at higher concentrations produced antagonistic effects (Bemis and Seegal 2000).
When challenged with 1.0 mg/kg AMPH prior to DRL testing, the performance of control males was more disrupted relative to all treated groups except the 3 mg/kg PCB + 4.5 ppm MeHg group. However increased total responding occurred in conjunction with increasing AMPH dose in all groups (see Table 4). Previous research examining the effects of developmental MeHg exposure has shown a similar AMPH effect on the DRL task (Eccles and Annau 1982). Generally with the DRL task, as AMPH dose increases, performance progressively worsens, while total responding increases at lower doses and decreases at higher doses (Sanger et al. 1974). The fact that these treatment groups were less disrupted by AMPH yet had increased total responding relative to non-drug days suggests a shift in the dose response curve for AMPH in the exposed males. Optimal performance on tasks such as DRL has been shown to be dependent upon moderate stimulation of dopamine (DA) receptors in the prefrontal cortex (PFC) (Russell 2002; Sokolowski and Salamone 1994). Either too much or too little DA activity can cause a disruption in performance. A reduction in DA activity has been found following developmental exposure to either PCBs (Seegal et al. 1997) or MeHg (Daré et al. 2003). Thus, DA hypofunctionality may have resulted in a lesser degree of overstimulation by AMPH in the exposed males relative to control males resulting in less impaired performance.
A number of researchers have commented that the inhibitory control deficits observed following developmental PCB exposure are similar to the impairments in response inhibition observed in animal models of ADHD (Berger and Sagvolden 1998) and in ADHD children (Sagvolden et al. 1998). The results of the current study provide additional support for this contention. Likewise, the results of the AMPH drug challenges suggest that the neurobehavioral effects of PCB exposure may be mediated by a reduction in prefrontal DA activity - a neurochemical profile that is also associated with animal models of ADHD (Russell 2002) and ADHD children (Dalley et al. 2008). While the PCB doses used in this study were significantly higher than would typically be encountered in children from environmental exposure, basic principles of allometric scaling dictate that rodents must receive much higher doses than humans in order to achieve comparable body burdens. Furthermore, the purpose of this study was to determine whether PCBs and/or MeHg impair response inhibition in a manner similar to that reported in humans (Stewart et al. 2006) and to begin to investigate the underlying neurochemical changes responsible for PCB- or MeHg-induced impairments in response inhibition, not to determine what concentrations of these chemicals would be sufficient to produce effects in humans. Additional studies are planned in our laboratory to further evaluate the effects of developmental PCB exposure on inhibitory control and attention (attentional impairments are also a core clinical symptom of ADHD). Follow up drug challenges using accepted ADHD pharmacotherapies will also be conducted to further examine the parallels between developmental PCB exposure and ADHD.
Acknowledgements
This research was funded by grants from the U.S. EPA (R-82939001) and NIEHS (PO1 ES11263, ES015687, and K99 ES15428). Paul Eubig was supported on T32 ES07326 during this project. The authors would like to Dr. Larry Hansen and Dr. Paul Kostyniak for their help in formulating the PCB mixture.
References
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environ. Health. Perspect. 1999;107:879–85. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury alter intracellular calcium concentrations in rat cerebellar granule cells. Neurotoxicology. 2000;21:1123–34. [PubMed] [Google Scholar]
- Berger DF, Sagvolden T. Sex differences in operant discrimination behaviour in an animal model of attention-deficit hyperactivity disorder. Behav. Brain. Res. 1998;94:73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Hussain RJ, Berger DF, Lombardo JP, Park HY. Electrophysiologic and behavioral effects of perinatal and acute exposure of rats to lead and polychlorinated biphenyls. Environ. Health. Perspect. 2002;110(Suppl 3):377–86. doi: 10.1289/ehp.02110s3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi AF, Blandini F, Randine G, Samuele A, Manzo L, Coccini T. Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2,2',4,4',5,5'-hexachlorobiphenyl (PCB 153) Brain. Res. 2006;1112:91–98. doi: 10.1016/j.brainres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys, and humans. Biol. Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Daré E, Fetissov S, Hökfelt T, Hall H, Ogren S, Ceccatelli S. Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003;367:500–508. doi: 10.1007/s00210-003-0716-5. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Wilding GE, Shamlaye CF, Huang LS, et al. Methylmercury and neurodevelopment: longitudinal analysis of the Seychelles child development cohort. Neurotoxicol. Teratol. 2006;28:529–535. doi: 10.1016/j.ntt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Feenstra MG, Broersen LM, van Leeuwen M, Arens C, de Vries S, Joosten RN. Role of the prefrontal cortex of the rat in learning and decision making: effects of transient inactivation. Prog. Brain. Res. 2000;126:103–113. doi: 10.1016/S0079-6123(00)26010-X. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev. Endocr. Metab. Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- Easton MDL, Luszniak D, Von der Geest E. Preliminary examination of contaminant loadings in farmed salmon, wild salmon and commercial salmon feed. Chemosphere. 2002;46:1053–1074. doi: 10.1016/s0045-6535(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Eccles CU, Annau Z. Prenatal methyl mercury exposure: II. Alterations in learning and psychotropic drug sensitivity in adult offspring. Neurobehav. Toxicol. Teratol. 1982;4:377–382. [PubMed] [Google Scholar]
- Faro LR, Rodrigues KJ, Santana MB, Vidal L, Alfonso M, Durán R. Comparative effects of organic and inorganic mercury on in vivo dopamine release in freely moving rats. Braz. J. Med. Biol. Res. 2007;40:1361–1365. doi: 10.1590/s0100-879x2006005000157. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Cada AM, Gray EP, Paule MG. No alterations in the performance of two interval timing operant tasks after alpha-difluoromethylornithine (DFMO)-induced cerebellar stunting. Behav. Brain. Res. 2001;126:135–46. doi: 10.1016/s0166-4328(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E, Reistad T. Molecular Mechanisms Involved in the Toxic Effects of Polychlorinated Biphenyls (PCBs) and Brominated Flame Retardants (BFRs) J. Toxicol. Environ. Health. A. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- Geller AM, Oshiro WM, Haykal-Coates N, Kodavanti PRS, Bushnell PS. Gender-dependent behavioral and sensory effects of a commercial mixture of polychlorinated biphenyls (Aroclor 1254) in rats. Tox. Sci. 2001;59:268–277. doi: 10.1093/toxsci/59.2.268. [DOI] [PubMed] [Google Scholar]
- Gerstenberger SL, Heimler I, Smies R, Hutz RJ, Dasmahapatra AK, Tripoli V, Dellinger JA. Minimal endocrine alterations in rodents after consumption of lake trout (Salvelinus namaycush) Arch. Environ. Contam. Toxicol. 2000;38:371–376. doi: 10.1007/s002449910049. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Rice DC, Burbacher TM. Fixed interval/fixed ratio performance in adult monkeys exposed in utero to methylmercury. Neurotoxicol. Teratol. 1996;18:539–546. doi: 10.1016/0892-0362(96)00081-5. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Rogan WJ. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. J. Pediatr. 1991;119:58–63. doi: 10.1016/s0022-3476(05)81039-x. [DOI] [PubMed] [Google Scholar]
- Goodwill MH, Lawrence DA, Seegal RF. Polychlorinated biphenyls induce proinflammatory cytokine release and dopaminergic dysfunction: protection in interleukin-6 knockout mice. J. Neuroimmunol. 2007;183:125–132. doi: 10.1016/j.jneuroim.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Burse WV, Needham LL, Storr-Hansen E, Heinzow B, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood toxicants. Neurotoxicol. Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Harer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol. Appl. Pharmacol. 1999;158:231–43. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Krogh H, Sagvolden T. Behavioural effects in female rats of postnatal exposure to sub-toxic doses of polychlorinated biphenyl congener 153. Acta. Paediatr. Suppl. 1999;88:55–63. doi: 10.1111/j.1651-2227.1999.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav. Brain. Res. 1998;94:213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr. 2003;143:780–8. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Kalisch BE, Racz WJ. The effects of methylmercury on endogenous dopamine efflux from mouse striatal slices. Toxicol. Lett. 1996;89:43–49. doi: 10.1016/s0378-4274(96)03787-3. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJ, Seegal RF, Pessah IN, Schantz SL. Formulation and Characterization of an Experimental PCB Mixture Designed to Mimic Human Exposure from Contaminated Fish. Toxicol. Sci. 2005;88:400–11. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lauber ME, Sarasin A, Lichtensteiger W. Sex differences and androgen-dependent regulation of aromatase (CYP19) mRNA expression in the developing and adult rat brain. J. Steroid. Biochem. Mol. Biol. 2007;61:359–364. [PubMed] [Google Scholar]
- Levin ED, Schantz SL, Bowman RE. Delayed spatial alternation deficits resulting from perinatal PCB exposure in monkeys. Arch. Toxicol. 1988;62:267–273. doi: 10.1007/BF00332486. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Neuf M, Munoz C, Winneke G. Behavioral effects of pre- and postnatal exposure to a mixture of low chlorinated PCBs in rats. Fundam. Appl. Toxicol. 1990;15:457–67. doi: 10.1016/0272-0590(90)90032-f. [DOI] [PubMed] [Google Scholar]
- Lyng GD, Snyder-Keller A, Seegal RF. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol. Sci. 2007;97:128–139. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- McKay SJ, Reynolds JN, Racz WJ. Effects of mercury compounds on the spontaneous and potassium-evoked release of [3H]dopamine from mouse striatal slices. Can. J. Physiol. Pharmacol. 1986;64:1507–1514. doi: 10.1139/y86-254. [DOI] [PubMed] [Google Scholar]
- Mele PC, Bowman RE, Levin ED. Behavioral evaluation of perinatal PCB exposure in rhesus monkeys: fixed-interval performance and reinforcement-omission. Neurobehav. Toxicol. Teratol. 1986;8:131–8. [PubMed] [Google Scholar]
- Minnema DJ, Cooper GP, Greenland RD. Effects of methylmercury on neurotransmitter release from rat brain synaptosomes. Toxicol. Appl. Pharmacol. 1989;99:510–521. doi: 10.1016/0041-008x(89)90158-0. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health . Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- National Research Council, Institute for Laboratory Animal Research . Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, D.C.: 2003. [Google Scholar]
- Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Miller RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34:231–41. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: effects on high- and low-rate operant behavior in adulthood. Neurotoxicol. Teratol. 2006;28:59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Powers BE, Poon E, Sable HJK, Schantz SL. Developmental exposure to PCBs, MeHg, or both: Long-term effects on auditory function. doi: 10.1289/ehp.0800428. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Prenatal methylmercury exposure increases responding under clocked and unclocked fixed interval schedules of reinforcement. Neurotoxicol. Teratol. 2007;29:492–502. doi: 10.1016/j.ntt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27:721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC. Effects of pre- plus postnatal exposure to methylmercury in the monkey on fixed interval and discrimination reversal performance. Neurotoxicology. 1992;13:443–52. [PubMed] [Google Scholar]
- Rice DC. Effect of postnatal exposure to a PCB mixture in monkeys on multiple fixed interval-fixed ratio performance. Neurotoxicol. Teratol. 1997;19:429–434. doi: 10.1016/s0892-0362(97)87364-3. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effects of postnatal exposure of monkeys to a PCB mixture on spatial discrimination reversal and DRL performance. Neurotoxicol. Teratol. 1998;20:391–400. doi: 10.1016/s0892-0362(97)00134-7. [DOI] [PubMed] [Google Scholar]
- Rice DC. Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environ. Health. Perspect. 2000;108(Suppl 3):405–8. doi: 10.1289/ehp.00108s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC, Hayward S. Effects of postnatal exposure to a PCB mixture in monkeys on nonspatial discrimination reversal and delayed alternation performance. Neurotoxicology. 1997;18:479–494. [PubMed] [Google Scholar]
- Rice DC, Hayward S. Lack of effect of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) throughout gestation and lactation on multiple fixed interval-fixed ratio and DRL performance in rats. Neurotoxicol. Teratol. 1998;20:645–650. doi: 10.1016/s0892-0362(98)00024-5. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain. Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Seo BW, Crofton KM, Schantz SL. Gestational-lactational exposure to Aroclor 1254 impairs radial-arm maze performance in male rats. Tox. Sci. 2000;57:121–130. doi: 10.1093/toxsci/57.1.121. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Wang VC, Powers BE, Klintsova AY, Villareal S, Greenough WT, Schantz SL. Motor impairment in rats exposed to PCBs and methylmercury during early development. Toxicol. Sci. 2004;77:315–24. doi: 10.1093/toxsci/kfg252. [DOI] [PubMed] [Google Scholar]
- Rogan JC, Keselman HJ, Mendoza JL. Analysis of repeated measurements. Brit. J. Math. Stat. Psych. 1979;32:269–86. [Google Scholar]
- Roselli CF. Brain aromatase: roles in reproduction and neuroprotection. J. Steroid. Biochem. Mol. Biol. 2007;106:143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav. Brain. Res. 2002;130:191–6. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Sable HJK, Powers BE, Wang VC, Widholm JJ, Schantz SL. Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicol. Teratol. 2006;28:548–556. doi: 10.1016/j.ntt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Sable HJK, Schantz SL. Executive function following developmental exposure to polychlorinated biphenyls (PCBs): What animal models have told us. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment (Frontiers in Neuroscience) CRC Press; New York, NY: 2006. pp. 147–167. [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav. Brain. Res. 1998;94:61–71. [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1239–47. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Key M, Blackman DE. Differential effects of chlordiazepoxide and d-amphetamine on responding maintained by a DRL schedule of reinforcement. Psychopharmacologia. 1974;38:159–171. doi: 10.1007/BF00426110. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ. Health Perspect. 2003;111:357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2',4'- and 3,4,3',4'-tetrachlorobiphenyl on dopamine function. Toxicol. Appl. Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain. Res. 1994;642:20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- Stewart J, Rajabi H. Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Res. 1994;646:157–160. doi: 10.1016/0006-8993(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol. Teratol. 2003;25:11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensititive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ. Health. Perspect. 2006;114:1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav. Brain. Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Crofton KM, MacPhail RC. Schedule-controlled behavior in rats exposed perinatally to the PCB mixture Aroclor 1254. Neurotoxicol. Teratol. 2002;24:511–8. doi: 10.1016/s0892-0362(02)00205-2. [DOI] [PubMed] [Google Scholar]
- van der Meulen JA, Joosten RN, de Bruin JP, Feenstra MG. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cereb. Cortex. 2007;17:1444–53. doi: 10.1093/cercor/bhl057. [DOI] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe P, Grandjean P, Debes F, White R. Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci. Total. Environ. 1996;186:141–8. doi: 10.1016/0048-9697(96)05094-2. [DOI] [PubMed] [Google Scholar]
- Weiss B, Landrigan PJ. The developing brain and the environment: an introduction. Environ. Health. Perspect. 2000;108(Suppl 3):373–4. doi: 10.1289/ehp.00108s3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicol. Appl. Pharmacol. 2001;174:188–98. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol. Teratol. 2003;25:459–71. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol. Sci. 2004;82:577–89. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton AD, Golden KM. Separation of drug effects on timing and behavioral inhibition by increased stimulus control. Exp. Clin. Psychopharmacol. 2000;8:451–61. doi: 10.1037//1064-1297.8.4.451. [DOI] [PubMed] [Google Scholar]