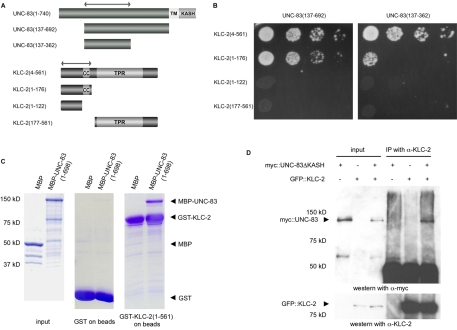
Fig. 1.
UNC-83 interacts with KLC-2. (A) Constructs of C. elegans UNC-83 and KLC-2 used for yeast two-hybrid and GST in vitro interaction analysis. The cytoplasmic portion of UNC-83 is dark gray. The transmembrane (TM) and KASH domains of UNC-83 and the coiled-coil (CC) and tetratricopeptide repeat (TPR) domains of KLC-2 are indicated. Double-headed arrows indicate the KLC-2-binding domain of UNC-83 and the UNC-83-binding domain of KLC-2. (B) Yeast two-hybrid interactions between UNC-83c (137-362) or UNC-83c (137-692) as bait and the indicated regions of KLC-2 as prey. Serial dilutions of log-phase cultures are shown. (C) Coomassie Blue stained SDS-PAGE gels demonstrating the interactions between GST-KLC-2 (1-561) and MBP-UNC-83 (1-698). In the left-hand panel, 1% of the input proteins are shown. The middle panel shows the amount of proteins pulled down by beads with GST only, to show background. The right-hand panel shows MBP and MBP-UNC-83 fusion proteins pulled down by GST-KLC-2 beads. Arrowheads mark the expected positions of the indicated constructs. (D) myc::UNC-83ΔKASH was co-immunoprecipitated with anti-KLC-2 antibodies. A western blot probed with anti-myc antibodies to recognize myc::UNC-83ΔKASH is shown in the top panel. Lanes 1-3 show transfected HeLa cell extracts used as inputs. Lanes 4-6 show immunoprecipitation by anti-KLC-2 antibodies from the same extracts. Beneath is shown the same blot probed with anti-KLC-2 antibodies. Size standards and the UNC-83 and KLC-2 fusion proteins are indicated on the left.