Abstract
Background
Surgical wounds are frequently contaminated by microbes, but rarely become infected if the bacterial burden is low, and irrigation is used to reduce contamination. Wound fluids are low in calcium and high in magnesium. We hypothesized that manipulating irrigant divalent cation concentrations might influence bacterial adhesion.
Methods
Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa were stained with fluorescent Calcein AM before plating onto fibroblast monolayers, collagen I, or uncoated bacteriologic plastic. After one hour, wells were washed with HEPES-buffered pH-balanced sterile water without or with 5mM CaCl2, 5mM MgCl2 or 1mM EDTA+EGTA, and the remaining adherent bacteria were assayed fluorometrically.
Results
Supplementing the irrigation with magnesium or chelators increased but calcium-supplemented irrigation reduced bacterial adhesion to collagen or fibroblasts. Non-specific electrostatic bacterial adhesion to uncoated plastic was unaffected by calcium.
Conclusion
Bacterial adhesion to mammalian cells and matrix proteins is influenced by divalent cations, and pathogenic bacteria may be adapted to adhere under the low calcium high magnesium conditions in wounds. Although these results await confirmation for other bacteria, and in vivo validation and safety-testing, they suggest that supplementing wound irrigation with 5mM CaCl2 may reduce bacterial adhesion and subsequent wound infection.
Keywords: Adhesion, bacteria, calcium, collagen, magnesium, infection, irrigation
INTRODUCTION
Open surgical wounds are frequently contaminated to some extent by various microbes despite standard surgical sterile technique, but in most cases the patient’s innate immune defense systems can eliminate the bacteria and prevent infection. The likelihood of infection is determined by a complex interplay between bacterial, host, and environmental factors, including the virulence of the bacteria, the degree of bacterial contamination, the surgical environment, perioperative management, and the host response. However, the adhesion of bacteria to the tissue represents the crucial first stage of the progression to infection. This does not mean that the mere presence of bacteria is the only critical issue. Bacteria create disease not only by their direct action but by secreting toxins, although if bacteria are washed out of a surgical wound before closure then they logically cannot continue to secrete toxins into the wound. However, even such bacterial pathogenic behavior may be governed by bacterial numbers by an incompletely understood process known as quorum sensing, in which bacterial density or concentration can trigger the release of factors which induce a pathogenic phenotype. Such quorum sensing has been described in Staph (1), E. coli (2), and Pseudomonas (3). Standards for operating room cleanliness, sterile technique, air exchange, and temperature have been defined, and guidelines have been established for the timing and appropriateness of prophylactic antibiotics, but less attention has been paid to methods to prevent bacterial adhesion.
Although we have not demonstrated directly in this paper that the degree of bacterial contamination correlates with the risk of wound infection, this concept underlies the classification of wounds as clean, clean-contaminated, contaminated, or dirty based upon the degree of contamination and the known increase in wound infection rates along this continuum. The more bacteria present in a wound, the higher the probability of wound infection (4). Thus, various authors have emphasized the importance of initial bacterial adhesion to tissues in generating subsequent infections (5) since if the bacteria do not adhere, they may be washed away. For instance, one study documented the dose-responsive nature of the risk of wound infection with Staph aureus or P. aeruginosa in an animal model (6) and furthermore suggested a cooperative and synergistic interaction between the two pathogens. The risk of wound infection with E. coli is similarly dose-dependent. For instance, a clinical study demonstrated that the risk of wound infection increases after elective laparotomy for inflammatory bowel disease proportionately to the degree of bacterial contamination with E. coli or Staph aureus in the operative field (7) while saline irrigation reduced the numbers of residual E. coli in guinea pig surgical wounds and reduced wound infection rates in parallel(8). Others have proposed laser light treatment of an open wound prior to closure to reduce the numbers of Staph aureus, E. coli, or P. aeruginosa in the wound (9).
Surgeons typically irrigate wounds and surgical sites with sterile water or saline. The mechanical force of such irrigation can remove necrotic debris, foreign material, and bacteria. Various antibiotics and antiseptic agents have been added to wound irrigation to kill residual bacteria with mixed results and in some cases potential toxicity. However, there has been less consideration of methods that might interfere with bacterial adhesion in a mechanistically directed fashion. Bacteria have many structures called adhesins that bind to different receptors found on specific host tissues (10). Bacterial capsules help to provide an outer layer covering to protect the bacterial cell from the extracellular slime(10). Most bacterial capsules are made of polysaccharides and proteins such as fimbriae and pili, which act as bacterial adhesins (10, 11). Adhesins in the form of lectins are more common and bind to specific saccharide receptors (11).
The precise mechanisms by which different bacteria adhere are incompletely understood. Some adhesion does occur by integrin heterodimers that contain a divalent cation binding site on their extracellular domain that when bound by magnesium or manganese upregulates integrin binding affinity (12). We have previously reported that calcium competes with magnesium or manganese for this site in mammalian integrin adhesion (13) presumably binding without invoking the degree of conformational change required for integrin activation. At least some other bacterial adhesion proteins may have homologous cation binding sites, but this awaits further study. We hypothesized that adding either divalent cation chelating agents or divalent cations, such as calcium and magnesium, would affect bacterial adhesion.
We studied three different gram positive or gram negative bacterial strains common in wound infections: Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli. To mimic the surgical situation in which bacterial contamination occurs during the procedure and the wound is subsequently irrigated before closure, bacteria were allowed to adhere to various substrates for one hour before being washed away by irrigation with sterile water that in some instances was supplemented with chelating agents, magnesium, or calcium. A HEPES buffer was added to all irrigants to maintain constant pH. In each case, we assessed adhesion to type I collagen, the dominant collagen of the interstitial extracellular matrix, and to monolayers of fibroblast GD-25 cells. We performed parallel studies of bacterial adhesion to bacteriologic plastic plates in the absence of physiologic substrates to assess whether the observed effects were modulating electrostatic or receptor-mediated bacterial adhesion.
MATERIALS AND METHODS
Bacteria
Staphylococcus aureus, Pseudomonas aeruginosa, and Escheria coli were obtained from ATCC (Manassas, VA). During the day prior to experimentation, an inoculated drop from a freezer stock of the bacterial strain to be studied was placed in 3ml of Luria Base Broth, LB Broth. The mixture of bacteria and LB Broth was incubated on a spinning table overnight for proper growth to be ready for experimentation the next day.
Cells
GD25 cells were obtained from the American Tissue Culture Collection. (ATCC, Manassas, VA) These cells were grown in GD-25 medium purchased from ATCC (Manassas, VA). Interstitial type I collagen also served as a substrate for adhesion.
Matrix precoating
Before plating the GD-25 cells, the twenty-four well plates were precoated with saturating concentrations of interstitial type I collagen (Sigma, St. Louis, MO) for 30 minutes in an ELISA-based coating buffer as previously described(13). After 30 minutes, the wells were washed with three times with sterile phosphate-buffered saline (PBS), and seeded with 100,000 GD-25 cells suspended in a total of 500 ul of GD-25 medium. The plates were then cultured to confluence prior to study. For studies of bacterial adhesion to type I collagen, twenty-four well plates were similarly precoated with a monolayer of type I collagen overnight at 4°C and washed three times with PBS before use.
Adhesion assay
After the bacteria were grown overnight, the suspension was measured in a UV/VIS Lambda 2 Spectrometer (Perkin Elmer, Waltham, Massachusetts) and diluted in LB broth to achieve an OD570 of 0.7, approximately 7.0 × 106 CFU per milliliter. The resulting bacterial suspension mixture of 8ml of LB broth and bacteria was then centrifuged in a Marathon 21000 R Centrifuge from (Fisher Scientific, Pittsburgh, Pennsylvania) at a speed of 2000 rpm or 800 g’s for 8 minutes at 4°C, the supernatant LB broth was removed, and the bacterial pellet was resuspended in 5ml of PBS and 5ul of Calcein AM, a fluorescent dye purchased from Invitrogen (Carlsbad, CA). Bacteria were incubated with the Calcein AM at 37°C for 15 minutes to permit bacterial staining, re-pelleted at a speed of 2000 rpm or 800 g’s for 8 minutes at 4°C, and resuspended in 7ml of LB broth with 10% FBS. The mixture of bacteria, LB broth, and 10% FBS was then agitated on an orbital shaker for 30 minutes at 37°C.
To prepare the monolayers, cells were washed three times with either pH-balanced sterile water with HEPES or a HEPES buffer supplemented with a divalent cation. Cells were then covered with a monolayer of 250ul of HEPES medium or HEPES medium supplemented with a divalent cation before addition of 250ul of bacterial suspension.
Bacteria were allowed to adhere to the substrates studied at 37 °C for one hour. After one hour, each well was washed with HEPES or HEPES supplemented with a divalent cation or chelating agent mixture three times. A FLX80C microplate fluorescence reader (Biotek Instruments, Inc., Winooski, Vermont) at an excitation level of 485nm/20nm was then used to quantitate the fluorescence of the remaining adherent bacteria in each well. Preliminary experiments demonstrated that this calcein AM fluorescent assay correlated closely with assays of bacteria counts by colony forming units (data not shown).
Data analysis
All experiments were conducted in twenty four well plates with n=6 and performed at least three times yielding similar results. Since all experiments yielded similar results, data were pooled for analysis after normalization to control values. Data were analyzed using the t-test seeking at least 95% confidence.
RESULTS
Chelation with EDTA and EGTA increases bacterial adhesion
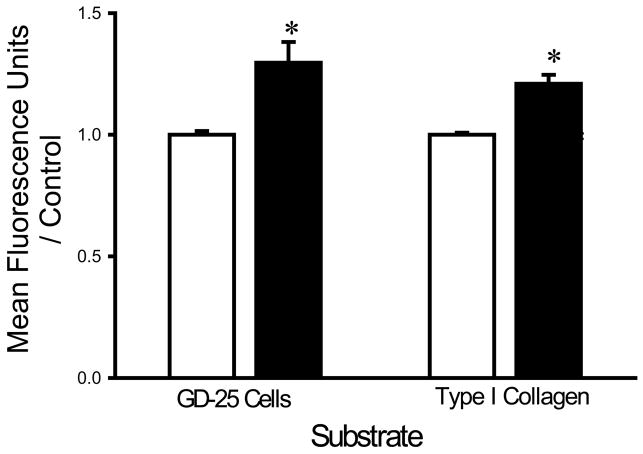
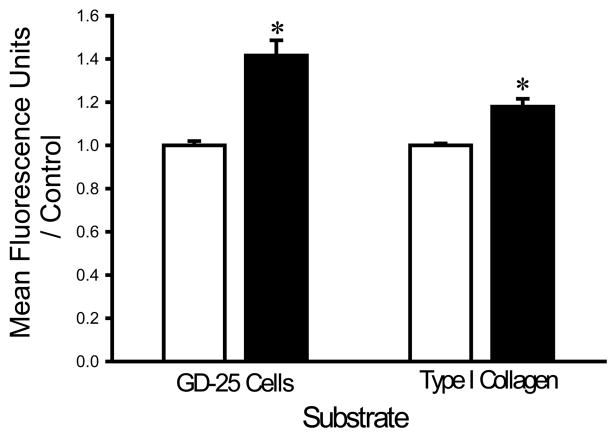
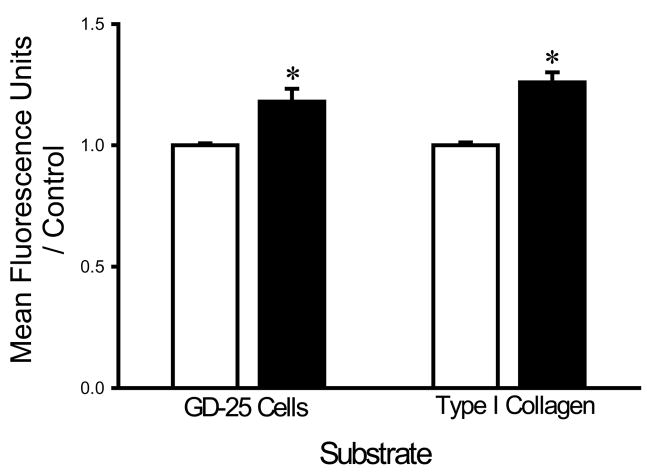
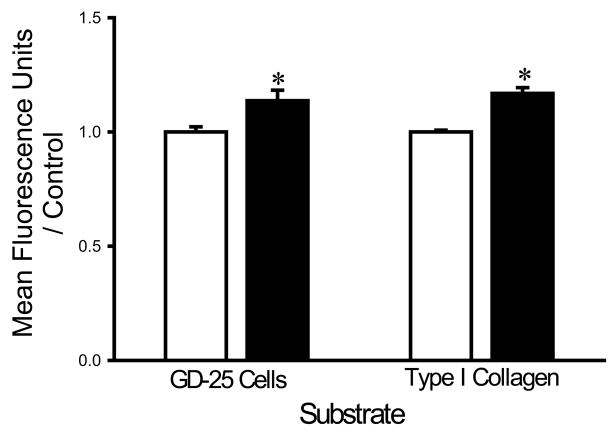
Washing with a HEPES buffer supplemented with 1 mM of EDTA and 1 mM EGTA increased the adhesion of each bacterial strain to fibroblasts and collagen I compared to washing with the same buffer without chelators. EDTA+EGTA increased S. aureus adhesion to fibroblasts and type I collagen by 36±8% and 21±4%, respectively (n=18, p<0.005 for each) (Fig. 1A). EDTA+EGTA increased P. aeruginosa adhesion to fibroblasts and type I collagen by 42±7% and 18±3%, respectively (n=18, p<0.00005 for each) (Fig. 1B). EDTA+EGTA also increased E. coli adhesion to fibroblasts and type I collagen by 14±7% and 21±3% (n=18, p<0.05), respectively (Fig. 1C).
Fig. 1.
Effect of chelating agents on bacterial adhesion. Irrigation with HEPES-buffered water supplemented with 1 mM EDTA+EGTA (shaded bars) increased adhesion of S. aureus (A), P. aeruginosa (B), and E. coli (C) to fibroblast GD-25 cells and type I collagen compared with irrigation with HEPES-buffered water without chelating agents (open bars). All data are normalized to bacterial adhesion. (n=18, *p<0.05)
Calcium decreases bacterial adhesion
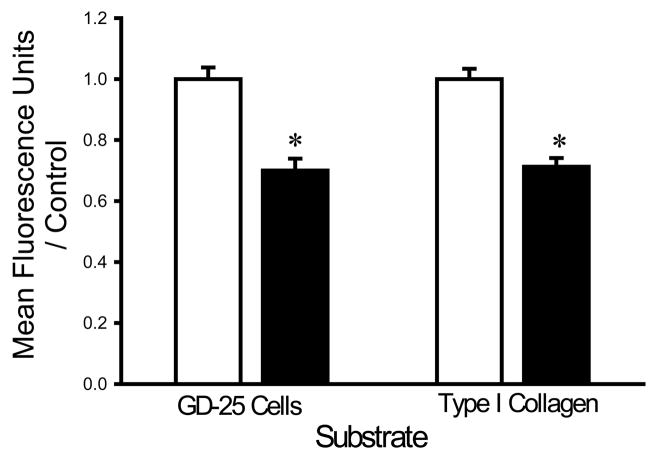
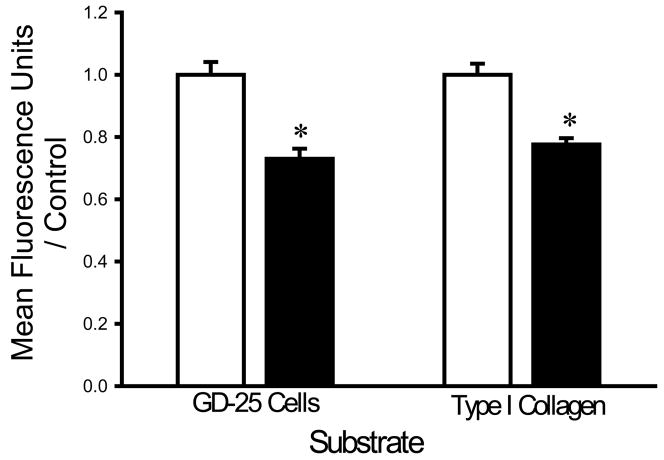
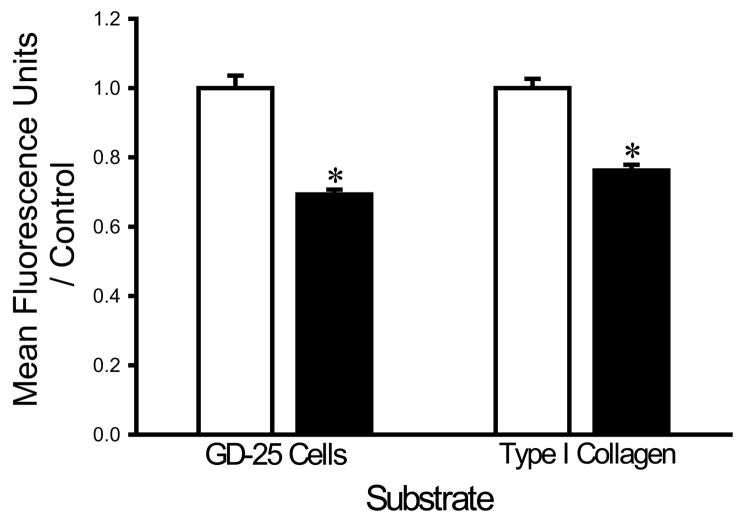
The finding that divalent cation chelation facilitated bacterial adhesion suggested that divalent cations might interfere with bacterial adhesion. We therefore next studied the effects of supplementing the irrigation buffer with 5mM Ca2+. The calcium-supplemented HEPES buffer decreased the adhesion of each bacterial strain. Calcium decreased the adhesion of S. aureus to fibroblasts and type I collagen by 30±4% and 29±3%, respectively (n=18, p<0.0005 for each) (Fig. 2A). E. coli adhesion to fibroblasts and type I collagen was similarly decreased by calcium by 27±3% and 23±2%, respectively (n=18, p<0.00001 for each) (Fig. 2B). The adhesion of P. aeruginosa to fibroblasts and type I collagen was also decreased by calcium by 31±2% and 24±2%, respectively (n=18, p<0.00001) (Fig. 2C).
Fig. 2.
Effect of calcium on bacterial adhesion. Calcium-supplemented irrigation (shaded bars) decreased adhesion of S. aureus (A), P. aeruginosa (B), and E. coli (C) to fibroblast GD-25 cells and type I collagen compared with irrigation with HEPES-buffered water without calcium (open bars). All data are normalized to bacterial adhesion. (n=18, *p<0.0005)
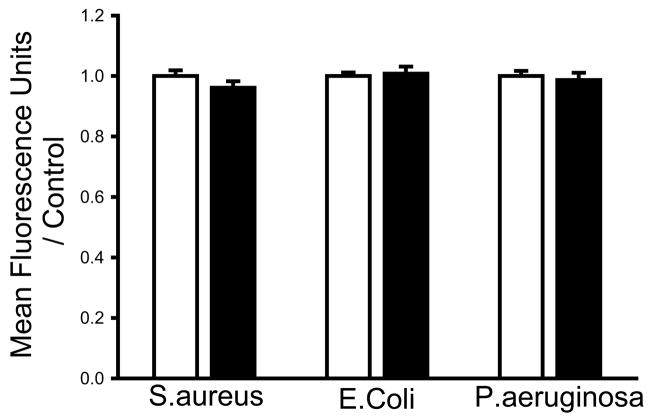
Bacterial adhesion to bacteriologic plastic is unaffected by calcium supplementation of the irrigant
Bacteria may adhere via specific receptors or simply via electrostatic effects. We studied bacterial adhesion to bacteriologic plastic, which involves only electrostatic effects(14, 15), to assess the specificity of the observation that calcium inhibits bacterial adhesion. We observed no significant change in adhesion of any of the bacterial strains to bacteriologic plastic when we washed with a calcium-supplemented HEPES buffer.
Magnesium increases bacterial adhesion
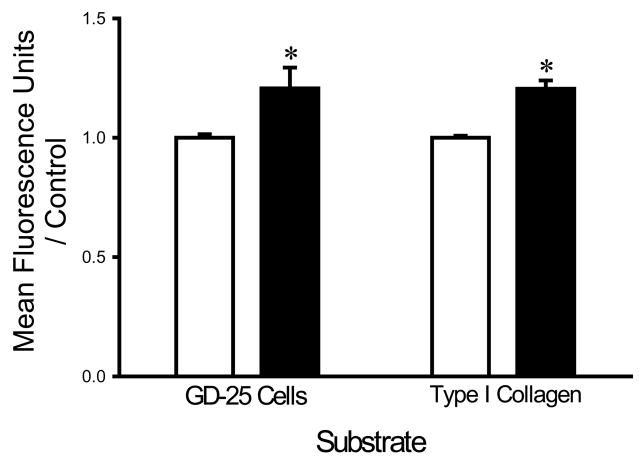
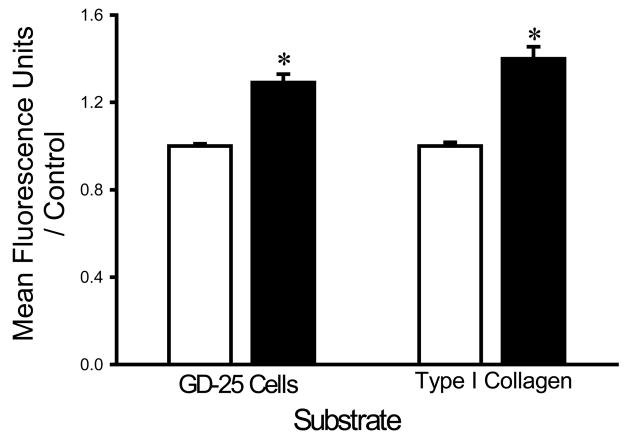
In contrast, to observations with calcium irrigation, supplementing the irrigation buffer with 5 mM Mg2+ increased the adhesion of each bacterial strain to all three substrates. S. aureus adhesion to fibroblast cells and type I collagen was increased in the presence of magnesium supplementation by 18±5% and 17±3%, respectively (n=18, p<0.005) (Fig. 4A). Similarly, E. coli adhesion to fibroblasts and type I collagen was increased by 18±5% and 29±4%, respectively (n=18, p<0.00001 for each) (Fig. 4B) and the adhesion of P. aeruginosa the same substrates was increased by 14±5% and 17±3%, respectively (n=18, p< 0.05 for each) (Fig. 4C).
Fig. 4.
Effect of magnesium on bacterial adhesion. Magnesium (shaded bars) increased adhesion of S. aureus (A), P. aeruginosa (B), and E. coli (C) to fibroblast GD-25 cells and type I collagen compared with irrigation with HEPES-buffered water without magnesium (open bars). All data are normalized to bacterial adhesion. (n=18, *p<0.05)
DISCUSSION
Wound infections, more recently classified as surgical site infections, account for about 14–16% of all infections that occur in the perioperative period in the United States (16). Surgical site infections are the major cause of postoperative death globally, accounting for about 77% of deaths of surgical patients (17). Although wound infections have a multifactorial etiology, it is clear that the risk of wound infection is directly proportional to the degree of bacterial contamination (18). This study suggests that chelation with the combination of EDTA and EGTA, or supplementation of irrigation buffer with magnesium increases the adhesion of the three bacterial strains studied to a variety of substrates, at least in vitro, but supplementing the irrigation with calcium may inhibit bacterial adhesion.
Many surgical procedures offer either shorter or longer time for bacterial adhesion than the one hour period studied here. Experimental standardization necessitated choosing a single time point for our studies. However, the adhesion of bacteria is at maximum during the first hour of adhesion and decreases thereafter because of a decrease in surface hydrophobicity (19). Thus, this one hour time point may be a good starting point to explore bacterial adhesion.
The first response to a bacterial infection in a wound is by the innate immune system of the body, which includes both the migration of inflammatory or phagocytic cells into the wound fluid and the release of more than 100 different peptide antibiotics that may be found in wound fluid and that may interfere with bacterial growth or adhesion. Although wound fluid would therefore seem likely to be bacteriostatic, this fluid also contains higher amounts of bacteria than wound tissues (20), presumably because not all bacteria successfully adhere and invade the tissues while the immune counterresponse is more active within the tissues. Interestingly, wound fluid also contains high amounts of magnesium and low amounts of calcium compared with plasma (21). The present results raise the possibility that although these high magnesium and low calcium levels may serve other purposes, they may actually promote bacterial adhesion. Since these effects are not seen when adhesion to bacteriologic plastic is studied, they seem more likely to reflect specific effects on bacterial adhesion receptors than perturbation of electrostatic interactions (14, 15, 22). Pathogenic bacteria may have evolved adhesion receptors that can specifically take advantage of these fluctuations in divalent cation levels. The precise characterization of these receptors awaits further study, as the molecular mechanisms of bacterial adhesion seem more heterogeneous and less well understood than those responsible for mammalian cell adhesion (11).
There are clearly manifest differences between bacterial adhesion to cells or proteins in a dish and bacterial adhesion to surgical wounds in vivo. These results therefore await extension to animal models before translation to humans. However, the effects that we observed were similar whether the substrate was type I collagen, the dominant collagen of the interstitial extracellular matrix that characterizes surgical wounds, or fibroblasts, common cells in open surgical wounds. Whether these results also apply to endothelial cells, important in open chronically granulating wounds, is beyond the scope of the current manuscript.
Calcium chloride is inexpensive and readily available in the operating room. Interestingly, our previous studies have also demonstrated that calcium irrigation can decrease the adhesion of mammalian cancer cells to matrix proteins (13) and surgical wounds, thereby promoting tumor-free survival in a murine transplantable tumor model (23). Although calcium may reduce bacterial adhesion to an open wound, another question is whether calcium might actually modulate bacterial virulence. This is clearly an open question awaiting more study. However, the absence of calcium actually triggers virulence in Pseudomonas, and Yersinia strains (24). Of course, other bacteria might respond differently to calcium, either for adhesion or pathogenicity, and this must await eventual study in a less controlled clinical setting to address the question of how the specific bacteria that cause wound infections in our patients might respond.
It should be emphasized that the present results await in vivo validation, and it will also be important to demonstrate that such interventions do not have other deleterious effects. Serum cation concentrations are not changed after murine wound irrigation (23), but humans may offer different pharmacokinetics, while possible interference with wound healing or other host responses to injury must still be ruled out. However the present results may increase the attraction of a strategy of irrigating surgical wounds with calcium-supplemented fluids after clean-contaminated resection of neoplasms such as those of colonic origin.
Fig. 3.
Adhesion to bacteriologic plastic. There were no significant changes in bacterial adhesion to bacteriologic plastic when plastic wells seeded with S. aureus, P. aeruginosa, or E. coli were washed with a calcium-supplemented HEPES buffer (shaded bars) compared with irrigation with buffer without calcium (open bars).
Acknowledgments
Supported in part by NIH RO1 DK060771 (MDB) and a Wayne State University 2008 Undergraduate Research Grant (CD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonetti O, Cirioni O, Ghiselli R, Goteri G, Scalise A, Orlando F, Silvestri C, Riva A, Saba V, Madanahally KD, Offidani A, Balaban N, Scalise G, Giacometti A. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:2205–2211. doi: 10.1128/AAC.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters M, Sperandio V. Quorum sensing in Escherichia coli and Salmonella. Int J Med Microbiol. 2006;296:125–131. doi: 10.1016/j.ijmm.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Heurlier K, Denervaud V, Haas D. Impact of quorum sensing on fitness of Pseudomonas aeruginosa. Int J Med Microbiol. 2006;296:93–102. doi: 10.1016/j.ijmm.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosch A, Lehnert H, Lobmann R. Microbiological aspects and antibiotic therapy of diabetic foot infections. Med Klin (Munich) 2003;98:259–265. doi: 10.1007/s00063-003-1254-0. [DOI] [PubMed] [Google Scholar]
- 5.Pruitt BA, Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 6.Hendricks KJ, Burd TA, Anglen JO, Simpson AW, Christensen GD, Gainor BJ. Synergy between Staphylococcus aureus and Pseudomonas aeruginosa in a rat model of complex orthopaedic wounds. J Bone Joint Surg Am. 2001;83-A:855–861. doi: 10.2106/00004623-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Claesson B, Brandberg A, Nilsson LO, Kock NG. Quantitative recovery of contaminating bacteria at operation and the relation to postoperative infection in intestinal surgery. Acta Chir Scand. 1981;147:285–288. [PubMed] [Google Scholar]
- 8.Badia JM, Torres JM, Tur C, Sitges-Serra A. Saline wound irrigation reduces the postoperative infection rate in guinea pigs. J Surg Res. 1996;63:457–459. doi: 10.1006/jsre.1996.0292. [DOI] [PubMed] [Google Scholar]
- 9.Lipovsky A, Nitzan Y, Lubart R. A possible mechanism for visible light-induced wound healing. Lasers Surg Med. 2008;40:509–514. doi: 10.1002/lsm.20668. [DOI] [PubMed] [Google Scholar]
- 10.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons RJ. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989;68:750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- 12.Baneres JL, Roquet F, Martin A, Parello J. A minimized human integrin alpha(5)beta(1) that retains ligand recognition. J Biol Chem. 2000;275:5888–5903. doi: 10.1074/jbc.275.8.5888. [DOI] [PubMed] [Google Scholar]
- 13.Thamilselvan V, Fomby M, Walsh M, Basson MD. Divalent cations modulate human colon cancer cell adhesion. J Surg Res. 2003;110:255–265. doi: 10.1016/s0022-4804(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 14.Terada A, Yuasa A, Kushimoto T, Tsuneda S, Katakai A, Tamada M. Bacterial adhesion to and viability on positively charged polymer surfaces. Microbiology. 2006;152:3575–3583. doi: 10.1099/mic.0.28881-0. [DOI] [PubMed] [Google Scholar]
- 15.McEldowney S, Fletcher M. Variability of the Influence of Physicochemical Factors Affecting Bacterial Adhesion to Polystyrene Substrata. Appl Environ Microbiol. 1986;52:460–465. doi: 10.1128/aem.52.3.460-465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. quiz 279–280. [DOI] [PubMed] [Google Scholar]
- 18.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dexter SJ, Pearson RG, Davies MC, Camara M, Shakesheff KM. A comparison of the adhesion of mammalian cells and Staphylococcus epidermidis on fibronectin-modified polymer surfaces. J Biomed Mater Res. 2001;56:222–227. doi: 10.1002/1097-4636(200108)56:2<222::aid-jbm1087>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Breuing K, Kaplan S, Liu P, Onderdonk AB, Eriksson E. Wound fluid bacterial levels exceed tissue bacterial counts in controlled porcine partial-thickness burn infections. Plast Reconstr Surg. 2003;111:781–788. doi: 10.1097/01.PRS.0000041540.22057.89. [DOI] [PubMed] [Google Scholar]
- 21.Grzesiak JJ, Pierschbacher MD. Shifts in the concentrations of magnesium and calcium in early porcine and rat wound fluids activate the cell migratory response. J Clin Invest. 1995;95:227–233. doi: 10.1172/JCI117644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.San Millan R, Elguezabal N, Regulez P, Moragues MD, Quindos G, Ponton J. Effect of salivary secretory IgA on the adhesion of Candida albicans to polystyrene. Microbiology. 2000;146 (Pt 9):2105–2112. doi: 10.1099/00221287-146-9-2105. [DOI] [PubMed] [Google Scholar]
- 23.van der Voort van Zyp J, Conway WC, Thamilselvan V, Polin L, Basson MD. Divalent cations influence colon cancer cell adhesion in a murine transplantable tumor model. Am J Surg. 2005;190:701–707. doi: 10.1016/j.amjsurg.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Rietsch A, Wolfgang MC, Mekalanos JJ. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect Immun. 2004;72:1383–1390. doi: 10.1128/IAI.72.3.1383-1390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]