Figure 1.
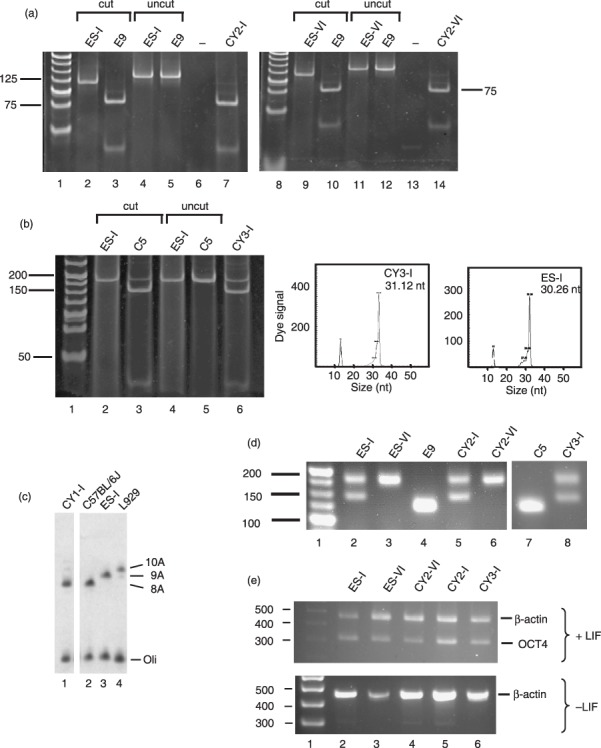
Production of transmitocondrial murine embryonic stem (ES) cell cybrids. (a) Transfer of mtDNA carrying a mild mutation in Mtco1 to pluripotent ES‐I and ES‐VI ES cell lines. A 133‐bp amplicon spanning nt6589 was generated from the indicated ES parental cells (ES‐I, lanes 2 and 4; ES‐VI, lanes 9 and 11), 6589T>C fibroblasts (E9, lanes 3, 5, 10 and 12) and ES cybrids (CY2‐I, lanes 7 and 14) as detailed in the Materials and methods and subjected to digestion with Cfo1. Cleavage generated a 115‐ and 18‐bp fragment for the wild‐type amplicon (lanes 2, 9), with further digestion of the 115‐bp fragment to 75 and 40 bp indicating the 6589T>C mutation (lanes 3, 7, 10 and 14). Uncut amplicon (lanes 4, 5, 11 and 12), negative control (lanes 6 and 13) and molecular weight markers (lanes 1, 8) are also shown. (b) Transfer of mutations in Mtnd5 and Mtnd6 to ES‐I. A 182‐bp amplicon spanning nt12273 was generated from ES parental cells (ES‐I, lanes 2 and 4), 12273G>A fibroblasts (C5, lanes 3 and 5) and ES cell cybrids (CY3‐I, lane 6) as detailed in the Materials and methods and subjected to cleavage with Dde1. Products carrying the mutation were cleaved to generate fragments of 145 and 37 bp (lanes 3 and 6). Uncut amplicon (lanes 4 and 5) and molecular weight ladder (lane 1) are shown. For analysis of mtDNA carrying the 13887C insertion, primer extension was performed as detailed in the Materials and methods. The panels show the length of extension corresponding to the normal mtDNA sequence (ES‐I, ~30 bp) or to the mtDNA carrying the single base insertion (CY3‐I, ~31 bp). The fluorescence signal due to the 5′ D3 fluorescent conjugate is indicated. The conjugated standard (13 nt) is also shown. (c) Transfer of the Mttr polymorphism to ES‐I. Primer extension was used to determine the length of the polyA tract around nt9821 in the mtDNA of various cell lines as detailed in the Materials and methods. Parental ES cells (ES‐I, lane 3), donor fibroblasts (C57BL/6J, lane 2) and cybrids (CY1‐I, lane 1) are shown, as well as the 10A extension found in L929 fibroblasts (lane 4). Radiolabelled oligonucleotide is also shown (oli). (d) Microsatellite markers confirm production of transmitochondrial cybrids. Amplicons spanning the informative marker D6mit102 were prepared from ES parental cells (ES‐I, lane 2; ES‐VI, lane 3), fibroblast donors (E9, lane 4; C5, lane 7) and cybrids (CY2‐I, lane 5; CY2‐VI, lane 6; CY3‐I, lane 8). Molecular weight markers are shown in lane 1. (e) Oct‐4 expression in parental and cybrid ES cells is dependent on leukaemia inhibitory factor (LIF). Cell lines (ES parental lanes 2, 3; cybrids lanes 4–6) were grown in the presence (+) or absence (–) of LIF for 9 days before RNA was isolated and reverse transcribed. Amplicons from the transcripts encoding β‐actin or Oct‐4 were generated as described in the Materials and methods. Molecular weight standards are given (lane 1).
