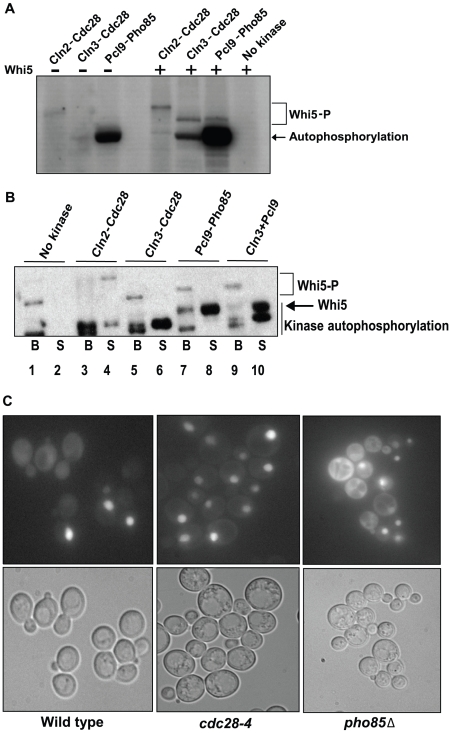
Figure 7. Pho85 does not affect known Whi5 regulatory mechanisms.
(A) Determination of relative Cdc28 and Pho85 kinase activity. In vitro kinase assays using varying amounts of recombinant Cln2-Cdc28, Cln3-Cdc28, and Pcl9-Pho85 in the absence (lane 1–3) or presence of purified Whi5 (lanes 5–8) were conducted and the degree of Whi5 phosphorylation was determined by SDS-PAGE and autoradiography. Purified Whi5 and γ-32P-ATP were incubated in the absence of kinase in lane 8, and lane 4 is empty. A 3 µM final concentration of Cln3-Cdc28 and Pcl9-Pho85 and a 60 nM final concentration of Cln2-Cdc28 give similar amounts of 32P-incorporation in Whi5, although phosphorylation by Cln2-Cdc28 caused Whi5 to migrate more slowly than Whi5 phosphorylated by Cln3-Cdc28 or Pcl9-Pho85. The concentration of kinase used in (B) was based on these experiments. (B) Cln3-Cdc28 and Pcl9-Pho85 do not influence Whi5-SBF complex stability. A preassembled recombinant Whi5-Swi4FLAG-Swi6 complex bound to anti-FLAG resin was incubated with Cln2-Cdc28, Cln3-Cdc28, Pcl9-Pho85, or both Cln3-Cdc28 and Pcl9-Pho85 in the presence of radiolabeled ATP. After washing, proteins in the bound and supernatant fractions were identified by autoradiography. (C) Subcellular localization of Whi5 in cdk mutant strains. Wt (BY263), pho85Δ (BY391), and cdc28-4 strains (BY465) expressing WHI5GFP from a methionine-repressible promoter (pBA1981) were examined for Whi5GFP fluorescence. Representative fields are shown.