Table 2.
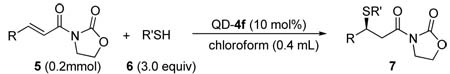
Conjugated addition of 6 to 5 with 4fa
 | |||||
|---|---|---|---|---|---|
| entry | R | R' | t(°C) | yield(%)b | ee(%)c |
| 1 | Me (5a) | Bn (6a) | −20 | 98 | 94 |
| 2 | Me (5a) | 4-ClBn (6b) | −20 | 99 | 93 |
| 3 | Me (5a) | 4-OMeBn (6c) | −20 | 99 | 96 |
| 4 | Me (5a) | C6H5CH2CH2(6d) | −20 | 98 | 92 |
| 5 | Me (5a) | TBSOCH2CH2 (6e) | −20 | 97 | 93d |
| 6 | Me (5a) | TESOCH2CH2 (6f) | −20 | 95 | 92 |
| 7 | Me (5a) | TMSCH2CH2 (6g) | −20 | 92 | 92 |
| 8 | Me (5a) | i-Bu(6h) | −20 | 91 | 90 |
| 9 | Me (5a) | Cyclopentyl(6i) | −20 | 96 | 94 |
| 10 | Me (5a) | Allyl(6j) | −20 | 97 | 94 |
| 11 | Et (5b) | 4-OMeBn (6c) | −20 | 98 | 91 |
| 12 | n-Pr (5c) | 4-OMeBn (6c) | −20 | 96 | 93 |
| 13 | i-Pr(5d) | 4-OMeBn (6c) | −20 | 98 | 93 |
| 14 | n-penta (5e) | 4-OMeBn (6c) | −20 | 96 | 95 |
| 15 | n-hex (5f) | 4-OMeBn (6c) | −20 | 97 | 94 |
| 16 | n-hepta (5g) | 4-OMeBn (6c) | −20 | 99 | 93 |
| 17 | C6H5(5h)e | 4-OMeBn (6c) | −50 | 95 | 87 |
| 18 | C6H5(5h)e | Allyl(6j) | −50 | 93 | 94 |
| 19 | 4-ClC6H5(5i)e | Allyl(6j) | −50 | 84 | 93 |
| 20 | 3-FC6H5(5j)e | Allyl(6j) | −50 | 94 | 92 |
| 21 | 2-BrC6H5(5k)e | Allyl(6j) | −50 | 91 | 96 |
Unless noted, reactions were carried out at −20 °C for 72 hrs.
Isolate yield;
Determined by HPLC analysis (see Supporting Information);
Absolute configuration of the 1,4-adduct 7 was determined to be R, for details, see supporting information;
catalyst loading : 20 mol%.
