Abstract
Context: Inferior petrosal sinus sampling (IPSS) best discriminates between the two causes of ACTH-dependent Cushing’s syndrome, Cushing’s disease (CD) and ectopic ACTH secretion (EAS). However, when sampling is not available, adjunctive diagnostic tests might be helpful. Neuroendocrine tumors may secrete chromogranin A (CgA), calcitonin (CT), procalcitonin (ProCT), a fragment of the amino terminus of procalcitonin (NProCT), and/or ACTH.
Objective: The aim of the study was to evaluate the ability of serum CgA, CT, ProCT, or NProCT values to distinguish CD from EAS.
Design and Setting: We conducted a prospective pilot study at a clinical research center.
Subjects and Methods: Serum ProCT, NProCT, and CgA were measured in six patients with occult EAS diagnosed by IPSS, 25 CD patients, and 11 patients with histologically proven EAS.
Results: Nine EAS patients (53%) had at least one value above the reference range, including CgA alone (n = 4), ProCT alone (n = 3), CgA and ProCT (n = 1), and NProCT and ProCT (n = 1). Of nine (36%) CD patients with one or two abnormal values, seven had increased ProCT only, one had increased NProCT only, and one had increased CgA and ProCT. CgA had a positive predictive value of 83% and a negative predictive value of 70% for the diagnosis of EAS; other markers showed less discrimination. On pituitary magnetic resonance imaging, no EAS patient had an abnormality, whereas 21 of 25 patients with CD had a mass.
Conclusion: These preliminary results suggest that an abnormal CgA and normal pituitary magnetic resonance imaging favor the diagnosis of EAS, but normal tumor markers do not exclude the diagnosis.
Measurement of chromogranin A, calcitonin, procalcitonin, or its amino terminus may provide additional tools for the differential diagnosis and management of patients with ACTH-dependent Cushing’s syndrome.
ACTH-dependent Cushing’s syndrome is caused by excessive ACTH secretion from a corticotroph tumor [Cushing’s disease (CD)] or a nonpituitary tumor [ectopic ACTH secretion (EAS)] (1). Because their clinical presentations may be similar, their distinction requires biochemical and imaging tests.
The diagnostic tests, CRH stimulation, 8 mg dexamethasone suppression, and inferior petrosal sinus sampling (IPSS) have up to a 10% or greater false-negative and false-positive rate (1,2) and are not universally available. Magnetic resonance imaging (MRI) of the pituitary gland has a sensitivity of 50% and a specificity of 94% for the diagnosis of CD (1). Thus, other tests might be useful. One possibility is that peptides secreted by neuroendocrine tumors (NETs) that do not produce ACTH might identify EAS patients.
Chromogranin A (CgA) identified 70 to 95% of non-ACTH secreting NETs (3,4) and seven of 10 patients with ACTH-producing NETs and a large tumor burden (5).
Calcitonin (CT) may be increased in patients with ACTH-secreting medullary thyroid carcinoma, small cell lung cancer, carcinoid, gastrinoma, and pheochromocytoma (6). Preprocalcitonin, its preprohormone, is cleaved to produce the prohormone, procalcitonin (ProCT), which includes an amino terminus (NProCT) (7). CT, ProCT, and NProCT may be increased in patients with non-ACTH NETs (8,9,10,11).
The present study tested whether excess NProCT, ProCT, or CgA would be more likely in EAS than CD.
Subjects and Methods
Inclusion criteria were current or previous hypercortisolism with plasma ACTH values greater than 10 pg/ml. Exclusion criteria included pregnancy, lactation, age less than 18 yr, and the presence of severe infection, plasma creatinine above 2.0 mg/dl (152.5 μmol/liter), severe heart failure, or cancer.
Consecutive patients were evaluated at the National Institutes of Health under institutional review board-approved protocols after providing informed consent. Each underwent pituitary MRI, CRH stimulation test, dexamethasone suppression test, and IPSS (1). All IPSS venograms were normal.
Patients received a provisional diagnosis of EAS or CD based on IPSS results (2). Patients with EAS underwent imaging for tumor localization.
Specimen collection and analytical methods
Hormones were measured within 6 d of tumor marker collection. For tumor markers, morning plasma was immediately separated and stored at −80 C until assay by individuals unaware of the patients’ diagnosis.
NProCT and ProCT were assayed at George Washington University. NProCT was measured using an ELISA assay [R2B7 antiserum developed in rabbits against synthetic human aminoprocalcitonin (Bachem, Torrance, CA)] at a final dilution of 1:80,000. The functional sensitivity is 20 pg/ml, and the 50% B/B0 is 240 pg/ml. The highest value in 13 healthy individuals was 77 pg/ml (10).
ProCT (Kryptor assay; BRAHMS, Henningsdorf, Germany) was measured by a sensitive, second-generation sandwich immunoassay that uses antibodies to CT carboxyterminal peptide-1 and CT (12). The intraassay precision is 10% at less than 100 pg/ml and 6% at 150 pg/ml. The interassay precision is 20% at less than 100 pg/ml and 8% at 150 pg/ml. The functional sensitivity is 60 pg/ml. The highest value in healthy adults was 60 pg/ml (12).
Serum CgA and CT were measured at Mayo Laboratories (Mayo, Rochester, MN) using immunochemiluminometric assays (ICMAs). The CgA assay uses antihuman CgA mouse monoclonal antibodies (13). The functional sensitivity is 5 ng/ml. The intraassay precision is 15.4% at 5.3 ng/ ml, and the interassay precision is less than 10% from 13–194 ng/ml. The reference range, less than 225 ng/ml (μg/liter), is based on results from 177 healthy adults, 144 children, and 86 pregnant women.
CT was measured by an automated ICMA using the Immulite 2000 analyzer (Siemens, Los Angeles, CA). The intraassay precision ranges from 4.7 to 2.9% between mean levels of 10.8 and 898 pg/ml, and interassay precision ranges from 6.4 to 3.4% between mean levels of 9.4 and 428 pg/ml. The functional sensitivity is 5.0 pg/ml. Based on 197 healthy adults, the reference range is less than 8.0 pg/ml (8 ng/liter) for females and less than 16 pg/ml (16 ng/liter) for males (14).
Urinary free cortisol was measured by Nichols Advantage one-site ICMA at the Department of Laboratory Medicine (DLM), National Institutes of Health, or by liquid chromatography tandem mass spectrometry at Mayo. Serum cortisol and plasma ACTH were measured by ICMA (IMMULITE 2500, Siemens Medical Solutions Diagnostics, Los Angeles, CA) at DLM.
Data capture and analysis
Patients’ age, gender, hormone concentrations, tumor size and histology, surgical findings, and size of MRI lesions were recorded.
Values of ProCT, NProCT, and CgA below the lower detection limits were analyzed as the numerical value of the limit. We used the highest values in healthy volunteers for NProCT and ProCT and the laboratory upper reference limits (above) for CgA and CT as the threshold for abnormal results.
Patients receiving proton pump inhibitors (PPIs) or H2-receptor antagonists within 3 months were excluded from CgA analysis.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of markers for the diagnosis of EAS, and their 95% confidence intervals (CI) were calculated (15,16).
An unpaired t test compared EAS and CD tumor marker concentrations. Spearman rank correlation examined the influence of hormones and tumor bulk on marker levels in hypercortisolemic patients. The relationship between tumor invasion and markers was evaluated by Fisher’s exact test and was a retrospective secondary analysis. Data are presented as mean ± sd. Logarithmic transformation was applied as needed. P values less than 0.05 were considered significant.
Results
Subjects
We prospectively studied 45 patients from February 2004 to April 2009; three were excluded (distant history of hypercortisolism; lack of surgical cure at transsphenoidal surgery; increased serum creatinine). Twenty-five patients (24 women) aged 40 ± 12 yr (range, 19–67 yr) underwent transsphenoidal exploration based on IPSS results. All had postsurgical hypocortisolism and ACTH-positive tumor. Twelve patients had dural or cavernous sinus tumor invasion, based on histopathological evidence and/or the surgeon’s assessment.
Seventeen patients (seven women) aged 48 ± 16 yr (range, 23–82 yr) had presumed EAS. One patient had unresectable metastasis of thymic neuroendocrine cancer. Ten others had imaging localization followed by resection of an ACTH-positive tumor (diameter, 6.5–30 mm) and hypocortisolism. Resected tumors were carcinoids (eight pulmonary and two thymic). Four patients had regional lymph node metastasis. Six patients have occult EAS.
MRI results
Two patients did not have MRI. Eight CD patients had masses less than 5 mm in diameter, and 13 had masses of at least 5 mm. No EAS patient had a pituitary mass.
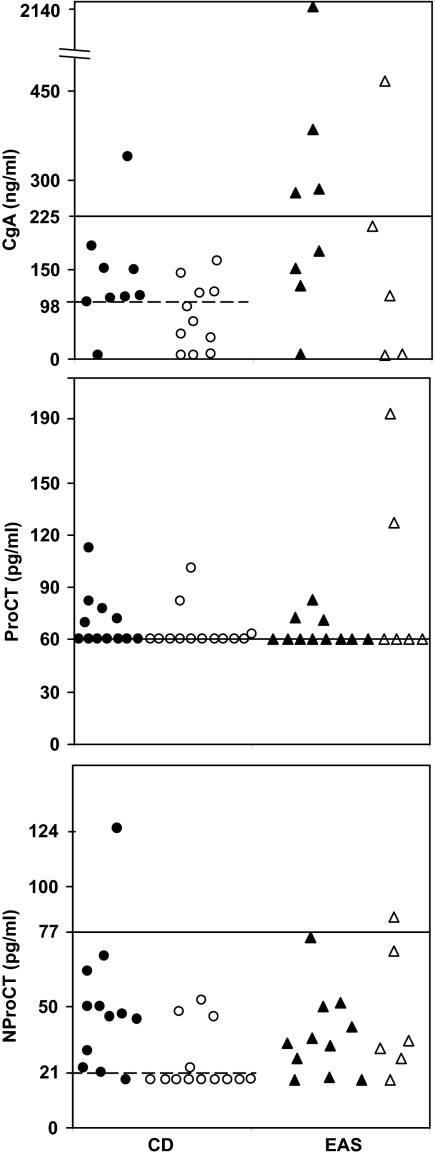
Biochemical results (Fig. 1)
Figure 1.
Scatterplots of CgA (top), ProCT (middle), and NProCT (bottom) levels in patients with CD with (•) and without (○) tumor invasion and in patients with EAS with identified tumors(▴) and occult tumors (▵). The horizontal line represents the upper normal limit for the assay. The dashed horizontal line represents the cutoff value in patients with CD that suggests the presence of an invasive tumor. The functional detection limits of the assay are 5 ng/ml (5 μg/liter) for CgA, 60 pg/ml for ProCT, and 20 pg/ml for NProCT.
The mean tumor marker concentrations were similar in the two groups.
CgA was elevated in five of 13 patients with EAS and one of 20 with CD not receiving PPIs or H2-receptor antagonists. CgA was the only abnormality in four EAS patients. The highest value was observed in a subject with metastatic thymic carcinoid (2140 ng/ml). Among patients receiving PPIs or H2-receptor antagonists, the mean CgA level was 532 ± 545 ng/ml [CD: n = 5; range, 5–1250 ng/ml; EAS: n = 4; 1953 ± 2912 ng/ml (range, 225-6280 ng/ml)].
In EAS, ProCT abnormalities were isolated in three patients and combined with CgA or NProCT (one each). Seven CD patients had only abnormal ProCT values. Another with abnormal ProCT (77.6 pg/ml) and CgA (340 ng/ml) had dural invasion.
Only two of 44 patients had an elevated NProCT: one patient with occult EAS had abnormal NProCT (84 pg/ml) and ProCT, and one with CD and dural invasion had elevated serum NProCT only (124 pg/ml).
Only one patient had a detectable CT value.
Relationships between tumor markers and hypercortisolism, tumor size, or activity
There was weak or no association between urinary free cortisol, cortisol or ACTH, or tumor size and tumor markers in any patient group (R2 < 0.36).
Tumor invasion of the dura or cavernous sinus was present in eight of 12 patients with CgA of at least 98 ng/ml and was absent in seven of eight with lower values (P = 0.028) (Fig. 1A). NProCT values also were different: 11 of 12 with, and four of 13 patients without invasive tumors had levels of at least 21 pg/ml (P = 0.0036) (Fig. 1C). ProCT was not associated with invasion (Fig. 1B). The tumor diameters of the patients with (4–15 mm) and without (3–10 mm) invasion were similar.
Tumor biomarker specificity and sensitivity, PPV, and NPV for the diagnosis of EAS (Table 1)
Table 1.
Sensitivity, specificity, PPV, and NPV values for diagnosis of EAS based on abnormal valuesa
| Abnormal marker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| CgA | 38 (18–64) | 95 (76–99) | 83 | 70 |
| ProCT | 29 (13–53) | 68 (48–83) | 38 | 59 |
| NProCT | 6 (1–27) | 96 (81–99) | 50 | 60 |
| One marker elevated | 53 (31–74) | 64 (44–80) | 50 | 67 |
| Two markers elevated | 12 (3–34) | 96 (80–99) | 67 | 62 |
Data within parentheses represent 95% CI.
No patient had elevation of all three markers.
CgA had the best diagnostic accuracy. The specificity increased and sensitivity for the diagnosis of EAS decreased when two abnormal results were required.
At least one marker was elevated in nine of 17 patients with EAS and in nine of 25 patients with CD. Using a combined criterion of an abnormal marker without an MRI abnormality, the PPV for EAS was 100%.
Discussion
This small study found that serum CgA concentrations worked better than ProCT and NProCT to distinguish between EAS and CD. CgA had a lower sensitivity (38%) than previous reports of non-ACTH secreting NETs (70–95%) (3). However, earlier studies included patients with a larger tumor burden (4,17,18). The highest concentrations of CgA were associated with hepatic thymic carcinoid metastasis. Altogether, these data suggest that serum CgA reflects tumor burden and/or access to blood supply.
Eight of 12 CD patients with dural invasion had CgA values of at least 98 ng/ml, and 11 had measurable NProCT values. This may reflect enhanced access to venous drainage via the cavernous sinus, although a fundamental difference in tumor biology cannot be excluded. Because no patient had an increased CT value, increased NProCT or ProCT values must reflect abnormal processing.
In previous reports, up to 50% of ectopic ACTH-producing tumors demonstrated immunoreactivity for more than one neuropeptide including CT and CgA (19,20). Based on this, we postulated that multiple abnormal peptides might provide stronger evidence for EAS. The specificity of any tumor marker abnormality increased to 96% when two abnormal values were required for the diagnosis of EAS, but sensitivity decreased dramatically.
This study has limitations. There may be a referral bias against overt EAS tumor (larger tumor mass) so that the frequency of elevated tumor marker concentrations may be falsely decreased. Because the study is small, the influence of age, gender, and confounding comorbidities on tumor markers could not be explored. Most patients had small nonmetastatic carcinoid tumors, whereas previous studies showed that the sensitivity of biomarkers depends on the tumor type and extent (3,4,5). Further investigations of patients with different types and extent of tumor burden are necessary to confirm and extend these findings and determine the best diagnostic criteria. The CT precursor assays are not widely available, thus decreasing their practical generalizability.
Our data suggest that measurement of tumor markers may offer clinicians additional tools for the differential diagnosis of patients with ACTH-dependent Cushing’s syndrome. Given the higher diagnostic accuracy of CgA, it should be preferred over other markers. Elevation of more than one marker provides evidence for EAS rather than CD. This information might be useful when other tests are inconclusive, equivocal, and/or not available. Finally, a CgA value of at least 98 ng/ml and/or NProCT value of at least 21 pg/ml in patients with presumed CD may be useful information for the management of these patients because they were associated with invasive tumors.
Footnotes
This work was supported, in part, by the Intramural Research Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 26, 2009
Abbreviations: CD, Cushing’s disease; CgA, Chromogranin A; CI, confidence interval; CT, calcitonin; EAS, ectopic ACTH secretion; ICMA, immunochemiluminometric assay; IPSS, inferior petrosal sinus sampling; MRI, magnetic resonance imaging; NET, neuroendocrine tumor; NProCT, amino terminus ProCT; NPV, negative predictive value; PPI, proton pump inhibitor; PPV, positive predictive value; ProCT, procalcitonin.
References
- Lindsay JR, Nieman LK 2005 Differential diagnosis and imaging in Cushing’s syndrome. Endocrinol Metab Clin North Am 34:403–421 [DOI] [PubMed] [Google Scholar]
- Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler Jr GB, Loriaux DL 1991 Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med 325:897–905 [DOI] [PubMed] [Google Scholar]
- Ferrari L, Seregni E, Bajetta E, Martinetti A, Bombardieri E 1999 The biological characteristics of chromogranin A and its role as a circulating marker in neuroendocrine tumours. Anticancer Res 19:3415–3427 [PubMed] [Google Scholar]
- Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW 1997 Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the α-subunit of glycoprotein hormones. J Clin Endocrinol Metab 82:2622–2628 [DOI] [PubMed] [Google Scholar]
- Nobels FR, de Herder WW, Kwekkeboom DJ, Coopmans W, Mulder A, Bouillon R, Lamberts SW 1994 Serum chromogranin A in the differential diagnosis of Cushing’s syndrome. Eur J Endocrinol 131:589–593 [DOI] [PubMed] [Google Scholar]
- Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK 2005 Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 90:4955–4962 [DOI] [PubMed] [Google Scholar]
- Becker KL, Nylén ES, White JC, Müller B, Snider Jr RH 2004 Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis—a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 89:1512–1525 [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Gicquel C, Lumbroso J, Tenenbaum F, Comoy E, Bosq J, Fonseca E, Ghillani PP, Aubert B, Travagli JP, Gardet P, Parmentier C 1992 Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest 15:631–642 [DOI] [PubMed] [Google Scholar]
- Becker KL, Snider RH, Silva OL, Moore CF 1978 Calcitonin heterogeneity in lung cancer and medullary thyroid cancer. Acta Endocrinol (Copenh) 89:89–99 [DOI] [PubMed] [Google Scholar]
- Snider Jr RH, Nylen ES, Becker KL 1997 Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med 45:552–560 [PubMed] [Google Scholar]
- Becker KL, Nylen ES, Cohen R, Snider RH 1996 Calcitonin: structure, molecular biology, and actions. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of bone biology. San Diego: Academic Press, Inc.; 471–494 [Google Scholar]
- BRAHMS PCT Sensitive KRYPTOR. Instruction for use 2008. www.procalcitonin.com [Google Scholar]
- Algeciras-Schimnich A, Preissner CM, Young Jr WF, Singh RJ, Grebe SK 2008 Plasma chromogranin A or urine fractionated metanephrines follow-up testing improves the diagnostic accuracy of plasma fractionated metanephrines for pheochromocytoma. J Clin Endocrinol Metab 93:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Preissner CM, Theobald JP, Finseth MS, Grebe SK 2009 Procalcitonin: a marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 94:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG 1998 Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17:857–872 [DOI] [PubMed] [Google Scholar]
- Motulsky H 1995 Interpreting lab tests: introducing to Bayesian thinking. In: Intuitive biostatistics. New York: Oxford University Press, Inc.; 129–136 [Google Scholar]
- Guignat L, Bidart JM, Nocera M, Comoy E, Schlumberger M, Baudin E 2001 Chromogranin A and the α-subunit of glycoprotein hormones in medullary thyroid carcinoma and phaeochromocytoma. Br J Cancer 84:808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose KJ, Scherag A, Hahmann M, Müller HH, Barth P 2008 Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol 6:820–827 [DOI] [PubMed] [Google Scholar]
- Liu TH, Liu HR, Lu ZL, Wang ZY, Cao Y, Chen J, Wang Y 1993 Thoracic ectopic ACTH-producing tumors with Cushing’s syndrome. Zentralblatt fur Pathologie 139:131–139 [PubMed] [Google Scholar]
- Gould VE, Lee I, Warren WH 1988 Immunohistochemical evaluation of neuroendocrine cells and neoplasms of the lung. Pathol Res Pract 183:200–213 [DOI] [PubMed] [Google Scholar]