Summary
Clinical trials are underway infusing T cells genetically modified to be specific for B-cell malignancies using a chimeric antigen receptor (CAR) to redirect specificity for CD19. However, issues remain regarding whether the CAR can provide a fully-competent application signal and whether other lymphocytes with lytic capacity can target CD19+ tumors.
In this issue of Clinical Cancer Research, Altvater et al. investigate whether a CD19-specific CAR (or chimeric receptor, chRec) can be generated to improve the ability of NK cells to target the CD19 molecule on the cell surface of malignant B cells, such as acute lymphoblastic leukemia (ALL).1 NK cells are an attractive cellular platform for combining gene therapy with immune-based therapy as they have endogenous cytolytic potential. This killing activity can be clinically harnessed by adoptively transferring haplotype-mismatched (haploidentical) NK cells which are capable of lysing acute myeloid leukemia (AML) blasts.2 This killing efficiently occurs upon mismatch between killer-cell immunoglobulin-like receptors (KIRs) and their ligands found on classical human leukocyte-antigen (HLA) B and C allele groups, and has been exploited to improve the graft-versus-leukemia (GVL)-effect after haploidentical hematopoietic stem-cell transplantation (HSCT), wherein engrafted alloreactive NK cells are attributed to target recipient AML blasts which lack KIR ligands (inhibiting HLA class I molecules) present on the donor-derived NK cells (“missing ligand”). 3 If engrafted haploidentical NK cells after HSCT are associated with anti-AML effect what if haploidentical NK cells were infused without HSCT? This has been tested and shown to be effective in some patients with refractory AML when haploidentical NK cells (peripheral blood depleted ex vivo of T cells and activated with IL-2) were infused after lymphodepleting chemotherapy and administered with IL-2, to improve the survival of the infused cells.4 We are building upon this success to infuse haploidentical NK cells for patients with solid tumors, such as neuroblastoma (ClinicalTrials.gov Identifier: NCT00698009)a. However, the clinical experience with NK cells targeting AML has not been duplicated for adult B-cell (B-ALL).
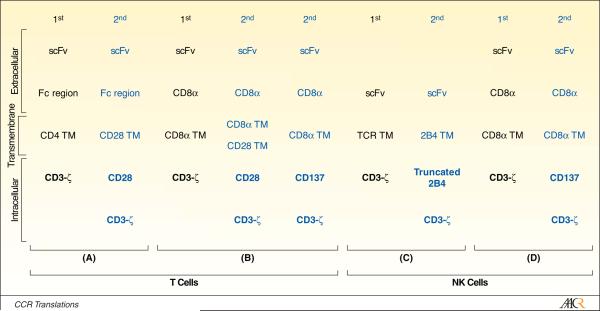
Rather than relying on the balance between endogenous activating and inhibitory receptors on NK cells to trigger cytolysis of tumor cells, investigators have genetically manipulated NK cells to express CARs to redirect specificity. CARs are typically derived from scFv region of a monoclonal antibody recognizing cell surface molecules, for example CD19. The prototypical CAR fuses the scFv exodomain with one or more intracellular chimeric signaling motifs to directly recognize tumor antigen, independent of HLA.5 Since much of the original work using CARs (aptly named “T-body”6) was redirecting the specificity of T cells, the endodomains have been tailored for T-cell signaling. For example, the “first generation” CAR that signals solely through CD3-ζ via its three immunoreceptor tyrosine-based activation motifs (ITAMs), has been coupled in subsequent generations to CD28, 4-1BBL and other co-stimulatory signaling cytoplasmic domains (Figure).7,8
Figure.
Schematic showing prototypical first (black font) and second (blue font) generation CD19-specific CARs that have been expressed in (A and B) T cells and (C and D) NK cells. The chimeric signaling endodomains are shown in bold type face on purple background. Not shown are “third generation” CARs which combine CD28 and CD3-ζ endodomains with chimeric CD137 (4-1BB), as well as other co-stimulatory domains. The CARs are derived from the following references: (A)8, (B)12, (C)1, (D)9. TM = transmembrane.
As CARs are evaluated in populations of lymphocytes other than T cells, it is probable that the nature of the cell to be manipulated will have to be considered to develop a CAR that is a fully-functional molecule capable of activating genetically modified cells for cytolysis, cytokine production and associated receptor upregulation, and proliferation. Previously, a first generation CD19-specific CAR was shown to activate NK cells through chimeric CD3-ζ and this signaling could be enhanced by the addition of a chimeric 4-1BB co-stimulatory endodomain.9 This work has now been expanded upon by Altvater et al. who generated a second-generation CAR (Figure) to improve NK-cell signaling by modifying their CD19-specific CAR endodomain to include a variant of the signaling lymphocyte activation molecule (SLAM)-related receptor 2B4 (CD244), which improves CAR-dependent activation of ex vivo-propagated NK cells in response to docking with CD19. The cell-surface molecule 2B4, which recognizes CD48, is endogenously expressed on NK cells, including this group’s NK cells that have been numerically expanded ex vivo on K562-derived artificial antigen presenting cells. The endogenous 2B4 co-receptor was capable of activating NK cells that express a first-generation CD19-specific CAR when targeting a CD19+CD49+ leukemia cell line. To coordinate signaling through CD3-ζ and 2B4 to target primary B-ALL blasts independent of CD48 expression, a next-generation CAR was built that signals through full-length 2B4 cytoplasmic domain fused to CD3-ζ (designated CD19-2B4ζ). By understanding that some of the 2B4 signaling domain’s four immunoreceptor tyrosine-based switch motifs (ITSMs) may provide deleterious signaling, the CD19-specific CAR was further modified to include a truncated 2B4 endodomain, shortened to just signal through the first two ITSMs, and fused to CD3-ζ (designated CD19-t2B4).
Just as autologous CAR+ T cells are currently being evaluated in clinical trials, pre-clinical data has been assembled to adoptively transfer NK cells expressing first and second generation CARs. Donor-derived NK cells may be attractive populations of cells to infuse after allogeneic HSCT to enhance the GVL-effect, for in contrast to T cells, engrafted allogeneic NK cells are not typically considered as instigators of graft-versus-host-disease.10 However, issues remain regarding the long-lived potential of genetically modified NK cells, and for that matter ex vivo propagated NK cells in general, to demonstrate sustained in vivo persistence and thus survive to exert a long term anti-tumor effect after adoptive transfer. Nevertheless, infusions of NK cells and CAR+ NK cells are now feasible to target both AML and B-ALL and clinical trials, perhaps concomitant with immune-modulating therapies,11 will determine whether these natural born killers can be called up in the war on cancer.
Footnotes
REFERENCES
- 1.Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling by recombinant CD19-specific chimeric receptors costimulates natural killer cell activation and cytolysis of acute lymphoblastic leukemia cells. Clin Cancer Res. 2009;15 doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 5.Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshhar Z, Bach N, Fitzer-Attas CJ, et al. The T-body approach: potential for cancer immunotherapy. Springer Semin Immunopathol. 1996;18:199–209. doi: 10.1007/BF00820666. [DOI] [PubMed] [Google Scholar]
- 7.Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 9.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron F, Petersdorf EW, Gooley T, et al. What is the role for donor natural killer cells after nonmyeloablative conditioning? Biol Blood Marrow Transplant. 2009;15:580–8. doi: 10.1016/j.bbmt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diermayr S, Himmelreich H, Durovic B, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111:1428–36. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 12.Milone MC, Fish JD, Carpenito C, et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol Ther. 2009 Apr 21; doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]