Introduction
Celiac disease (CD) is a common immune-mediated enteropathy with a prevalence of approximately 1% within the U.S. and European populations. There is however a world-side disease distribution, including Mexico, South America, the Middle East, parts of India, and specific regions of Africa. While the classic and usually obvious consequences of the enteropathy are malabsorption with diarrhea, weight loss, and nutritional deficiencies, the difficulty in diagnosis is due in large part to the silent form of the disease which comprises the majority of patients. Overall mild clinical symptoms with nonspecific complaints such as fatigue, headaches, arthralgias are common and can delay diagnosis. A large, multi-center study in the United States showed an increased disease prevalence in high risk groups, including patients with autoimmune insulin-dependent diabetes mellitus, Sjogren's syndrome, osteoporosis, and first degree relatives of patients with CD; in addition to diarrhea, abdominal pain and constipation were among the most highly reported symptoms. [1] Because of the diagnostic difficulties inherent in this often mild or clinically silent presentation, identification of high risk groups for serological screening may decrease time to diagnosis and lessen the complications of untreated disease.
Several studies have recently demonstrated the cost-effectiveness of screening the IBS population for CD. Results from one recent study addressed the possibility of immunologically-based mechanisms following gluten exposure contributing to IBS symptoms that may represent a ‘celiac-like’ disorder. This study showed decreases in stool frequency and improvement in the gastrointestinal symptoms score among 60% of patients with diarrhea-predominant IBS and who carried the celiac disease associated HLA type (DQ2), but lacked fully developed celiac disease. [2]
Various autoimmune diseases have been historically associated with CD. [3, 4] These findings raise interesting questions as to whether abnormal immune responses at the level of the gut mucosa when exposed to environmental antigens, play a role in systemic autoimmune disease or if these associations reflect more an underlying, genetic predisposition. Proposed mechanisms of association include abnormal regulation of intestinal permeability and increased autoantibody production in the setting of chronic gut inflammation.
This review will focus on the autoimmune connective tissue diseases, endocrine, and dermatological conditions associated with CD, as well as the related gut inflammatory disorders of refractory celiac disease, autoimmune enteropathy, collagenous enteritis, and collagenous colitis.
Gut Immunogenesis of Celiac Disease
Celiac disease is the result of an unchecked immune reaction to gluten. This unchecked response results in inflammation of the proximal small intestine where the partially digested gluten proteins contact the gut immune system. This immune response extends beyond just a direct response to the exogenous substance, but also includes a potent and multi-faceted immune response to autoantigens that results in substantial damage to the structure and function of the gut and other organs. The clinical manifestations are heterogenous, including complete absence of gastrointestinal complaints, a myriad of extraintestinal manifestations, signs of overt malabsorption, and in rare cases development of ulcerative jejunitis and enteropathy associated T-cell lymphoma. In the small intestinal mucosa CD4+ T-cells are stimulated by gliadin peptides only when presented by DQ2 or DQ8 MHC molecules on the surface of antigen-presenting cells. This binding triggers proliferation of pathogenic gluten-specific T-cells, a potent Th1 inflammatory response characterized by high levels of IFNγ, and subsequent villous atrophy. In addition to this response to gluten, there is a potent and almost universal humoral response to the autoantigen tissue transglutaminse in the gut. The role and indeed the production of tissue transglutaminase autoantibodies is not fully understood, but what is truly curious is the ability of cell-surface tissue transglutaminase to bind and deamidate gliadin peptides. [5] These deamidated peptides subsequently bind tightly to DQ2/DQ8 molecules potentiating CD4+ T-cell activation and proliferation. What the antibodies which are directed against the active site of the transglutaminase are doing is uncertain from a pathological perspective. Both T and B cell responses diminish upon withdrawal of gluten and normal villous architecture is restored.
Whilst antibodies directed against the wheat proteins have been pursued for many years, it was the advent of the much more specific antibodies directed against extracellular antigens that has revolutionized the detection of celiac disease. Given the high specificity of positive anti-endomysial (EMA) antibody titers and high sensitivity of IgA tissue transglutaminase (tTg), both tests in conjunction have a very high positive predictive value. EMA testing increases expense and is prone to interpretation errors, so tTg antibody testing alone is recommended for screening. Subsequent tTg antibody titers can be followed to assess occult gluten exposure or dietary indiscretion. Time to normalization of IgA tTg titers in the setting of a strict gluten free diet is variable, usually from weeks to months.
While it has been widely hypothesized that there may be environmental triggers for many autoimmune diseases, celiac disease is unique in that we know the major and necessary precipitating factors. Additionally, CD is different from most autoimmune diseases in that the environmental triggers need to be there all of the time or the disease and the autoantibodies regress. Most people with the known genetic predisposition (DQ2 or 8) and who eat gluten do not get CD, so there must be other factors. These additional factors contributing to disease development are less well understood, but likely include stimulation of an innate immune response to some environmental factor either derived from gluten or some other factor present in the intestinal lumen.
Environmental Factors
The timing or amount of gluten exposure may be important factors in disease development. The data to support this comes largely from observational studies. In the 1980s the incidence of CD among Swedish children increased significantly and remained high for approximately 10 years. Subsequently, with nationwide changes in infant feeding practices to reduce gluten exposure and alter its timing the incidence of celiac disease has dropped back to pre-epidemic levels. This “Swedish epidemic” generated the hypothesis that the introduction of large quantities of gluten after the cessation of breast feeding was responsible for the failure of development of immune tolerance to gluten in these young children. Breastfeeding feeding as a single factor did not appear to be the major risk factor. Dietary gluten exposure earlier than 3 months or later than 6 months of age was found to be a risk factor in a longitudinal follow up study of a cohort of children in Denver, CO. [6] These studies taken together suggest that there is a crucial window when tolerance to gluten occurs and overlapping the introduction of gluten with breastfeeding may provide the best protection against childhood celiac disease. Whether such a practice will prevent the lifetime development of celiac disease or another autoimmune disease ultimately is not known.
Ivarsson et al. found that risk of CD development increases with the number of gastrointestinal infections before six months of age and among infants born between the months of March and July; this seasonal risk was consistently shown year to year throughout the ten year epidemic. [7, 8] Significant interest in rotavirus as a possible infectious trigger was generated following a study by Stene et al. showing that frequent rotavirus infections increased the risk of CD development in 54 cases of children under four years of age. [9] Additional case reports of CD development in children following rotavirus infection have been published. Of recent interest are the roles of IL-15 and gastrointestinal flora in the proximal small bowel in relation to precipitating disease. [5, 10] The dysregulation of immune mechanisms in response to food and microbial antigens and how these mechanisms relate to gastrointestinal and systemic autoimmune diseases are the focus of ongoing research.
Risk of autoimmune disease
A 1999 study by Ventura et al. demonstrated an increased prevalence rate of autoimmune disease among 909 celiac patients as compared to controls, though no significant difference when compared to 163 Crohn's disease patients. [11] Logistic regression analysis showed that the age of CD diagnosis was a significant predictor of autoimmune disease development later in life. Follow-up studies addressing the length of gluten exposure as a predictor of autoimmune disease by Sategna-Guidetti et al. and Biagi et al. did not confirm these findings however. [12, 13] Cataldo and Marino showed higher prevalence of autoimmune disease (4.8%) among first degree relatives of celiac patients as compared to first degree relatives of healthy controls (0.86%). [14] The authors diagnosed a subset of these first degree relatives of CD patients with silent CD; a higher prevalence of autoimmune disease among this group was found as compared to healthy first degree relatives of CD patients, 20% vs. 3.8% respectively. They suggest this increased risk relates to the higher prevalence of silent CD. Whether untreated celiac disease is responsible for an incremental incidence in autoimmune diseases is unknown, though it is worthwhile for the clinician to be aware of these associations lest they occur. Generally, patients with newly diagnosed celiac disease can be reassured that their risk of developing another autoimmune disease is not greatly elevated after diagnosis and treatment.
Associations with Autoimmune Disease
Sjogren's syndrome (SS)
Several studies have revealed a high prevalence of CD among patients with SS. Iltanen et al. showed that 14.7% of 34 SS patients had CD confirmed by small bowel biopsy. [15] A more recent study from Hungary with 111 SS patients found the CD prevalence rate to be 4.5 per 100 confirmed by small bowel biopsy. [16] Luft et al. measured levels of tissue transglutaminase (tTg) autoantibodies in the sera of systemic lupus erythematosis (SLE), SS, and rheumatoid arthritis (RA) patients. [17] 12% of SS patients were positive for IgA tTg autoantibodies vs. 4% of controls. 5 of 6 patients with positive IgA tTg titers had villous atrophy on small bowel biopsy confirming the CD diagnosis. Significantly lower levels of tTg autoantibodies were found in SLE, RA, and systemic sclerosis patients.
Inflammatory Arthritis
There have been several studies seeking CD in cohorts with arthritis. Stagi et al. found an increased prevalence of CD among 151 children with juvenile idiopathic arthritis (6.7% vs. 0.6% controls). [18] Prevalence rates reported from Lepore et al. demonstrated increased prevalence of biopsy confirmed CD (2.5%) among 119 children with juvenile chronic arthritis (JCA). [19] This association however does not extend to adult rheumatoid arthritis (Table 1). [20]
Table 1.
Prevalence of CD among arthritis patients.
| Autoimmune disorder (#patients) | Age (yrs) | CD prevalence | CD diagnosis confirmation | |
|---|---|---|---|---|
| Stagi 2005 [18] | Juvenile idiopathic arthritis (151) | 2.4 to 16.9 (median 8.3) | 6.7% | Positive anti-endomysial and IgA tissue transglutaminase serology, SBBx |
| Lepore 1996 [19] | Juvenile chronic arthritis (119) | 2 to 16 (mean 11.5) | 2.5% | Positive anti-endomysial serology, SBBx |
| Francis 2002 [20] | Rheumatoid arthritis (60) | 20 to 84 (mean 61) | 0.63% | Positive anti-endomysial serology. SBBx not reported. |
| Autoimmune disorder (#patients) | Age (yrs) | Non-erosive arthritis prevalence | Effect of gluten-free diet (GFD) | |
| Lubrano 1996[21] | Previously diagnosed CD patients (200) | 18 to 65 (mean 32.8) | 26% | Higher prevalence of arthritis on regular diet 41% vs. 21% on GFD |
SBBx=small bowel biopsy
The prevalence of rheumatological conditions is not convincingly elevated in the celiac disease population as a whole. There have been several small case series that show that treatment with a gluten free diet may be helpful in some patients with musculoskeletal symptoms. Lubrano et al. demonstrated a 26% prevalence of arthritis among 200 adult CD patients. [21] The prevalence of arthritis was significantly higher and more severe among the group on a regular diet as yet untreated vs. those already established on a gluten free diet for an average of 58 months. This confirmed an earlier, small case series that showed improvement of the joint symptoms in newly diagnosed celiac disease patients following treatment with a gluten free diet. [22] The mechanisms underlying these arthritic complaints in CD are unknown though the alterations in intestinal permeability in untreated CD may be factor.
There have been a few case reports of clinically overt CD in systemic lupus erythematosis (SLE) patients. [23] The CD diagnosis consistently preceded the SLE diagnosis by an average 15 years in the pediatric cases and of the adults, the diagnosis of CD consistently followed the diagnosis of SLE by an average of 3.8 years. [23] SLE association to CD is likely rare as one study did not find hidden cases in 103 patients with SLE. [24] A more recent study from Northern Ireland did not reveal clinical evidence of SLE among a cohort of 60 celiac disease patients. [25] While gastrointestinal manifestations of SLE are common in both adults and children, [26-28] CD is relatively rare among this patient population but should be considered in the setting of malabsorption symptoms. False positive ANA tests may be found in patients with untreated celiac disease.
Associated Autoimmune Endocrine Diseases
Addison's disease
European studies estimate the prevalence of CD among patients with autoimmune Addison's disease to be between 1.2 – 8%. [29] More recent studies have demonstrated higher prevalence rates. A study from Ireland found 5 cases of celiac disease among 41 Addison's patients. [30] A recent, large Italian study showed among 109 patients with autoimmune Addison's disease (AAD), a 5.4% prevalence of CD vs. 0.06% among controls. [31] This association was confirmed by the analysis of greater than 14,000 celiac patients from the Swedish national register, showing an increased risk of developing Addison's disease among celiac patients. [32] Table 2 summarizes these recent studies.
Table 2.
Prevalence of CD among Addison's disease patients.
| Addison's disease (#patients) | Age (yrs) | CD prevalence | CD diagnosis confirmation | |
|---|---|---|---|---|
| O'Leary 2002[30] | 41 | 7 to 66 (mean 37) | 12.2% | Positive IgA tTg and anti-endomysial serology, SBBx confirmation |
| Myhre 2003 [78] | 76 | 6 to 85 (mean 45.6) | 7.9% (6 of 76, 1 patient with known CD) | Positive IgA tTg and anti-endomysial serology, SBBx confirmation |
| Biagi 2006 [79] | 17 | 26-79 (mean 53.9) | 5.9% | Positive anti-endomysial serology, SBBx confirmation |
| Betterle 2006 [31] | 109 | 11 to 87 (mean 42.7) | 5.4% (6 of 109, 2 patients with known CD) | Positive tTg IgA, 3 of 4 patients confirmed by SBBx (1 Silent CD, 2 Latent CD). |
| Celiac disease (#patients) | Age (yrs) | Risk (hazard ratio) | ||
| Efstrom 2007 [32] | Previously diagnosed CD patients (14,366) | 18 to 65 (mean 32.8) | 11 fold increased risk of developing Addison's disease |
SBBx=small bowel biopsy
AIDDM
Autoimmune insulin-dependent diabetes mellitus (AIDDM) has a high prevalence rate among the CD patient population. Serological screening for CD in AIDDM populations showed a median prevalence of 4.1% among 40 studies; these included primarily European centers as well as 6 centers from the USA and UK. [33] Gastrointestinal symptoms attributed to celiac disease among AIDDM patients are generally mild. Active malabsorption may lead to unreliable carbohydrate absorption and hence unpredictable glucose responses to meals. This can lead to some degree of brittleness in diabetic control. Folate and iron deficiency are also commonly seen. CD diagnosis often follows within one year of the AIDDM diagnosis. [33]
It is has not been universally accepted that all AIDDM patients should be screened for celiac disease, though some advocate screening for CD at intervals in children with AIDDM due primarily to the prevalence and the potential consequences of delayed diagnosis. [34] While it is rational to expect that early treatment would prevent complications of CD in this population there is little actual data that documents such benefit. There may be a slight increase in insulin total dose with improved absorption that comes with correction on the intestinal malabsorption. Certainly physicians caring for patients with AIDDM should have a very low threshold for celiac disease testing.
Thyroid disease
A recent review of thyroid disease studies revealed a high prevalence of CD among patients with autoimmune thyroid disease, Hashimoto's disease, Graves' disease, and pediatric autoimmune thyroid disease ranging from 2 to 7.8% (average 4.1%). [35] Occurrence of hyperthyroidism in adult celiac disease patients ranges from 0.1 to 5.2% (average 2.3%), while occurrence of hypothyroidism ranged from 0 to 5.8% (average 2.6%). [33] Whilst there is not sufficient data available to advocate screening for CD in patients with thyroid disease a much greater awareness should lead to a low threshold for testing. An awareness of the converse relationship is also crucial, as thyroid disease may explain fatigue and additional constitutional symptoms in treated CD.
Associated Autoimmune Liver Disease
Mild transaminitis in the absence of primary liver disease can occur in the setting of untreated CD; patients are often asymptomatic and develop mild periportal inflammation that resolves with a gluten free diet. Multiple studies and case reports have demonstrated variable associations between primary biliary cirrhosis, autoimmune hepatitis, primary sclerosing cholangitis and CD. With the exception of two negative studies, consistent association between PBC and CD has been shown. [36,37] In a recent review of CD and liver disease, percentage of biopsy confirmed CD cases among the PBC population ranged from 1.3 to 7%. [38] The largest study included greater than 13,800 CD patients from the Swedish national register and confirmed the association. [39]
Prevalence rates of CD among patients with autoimmune hepatitis (AIH) range from 4 to 6.4%. [40,41] Several other autoimmune diseases are more commonly associated with AIH including rheumatoid arthritis, synovitis, Grave's disease, autoimmune thyroiditis, and ulcerative colitis. [42] The association between AIH and CD appears to be less common in children than adults. [43]
Initially reported in 1988, the association between CD and PSC has been investigated in few large scale studies. Ludvigsson et al. recently published one of the largest studies utilizing the Swedish national register with the same cohort of greater than 13,800 CD patients, showing a 4 to 8 fold increased risk of PSC.
Testing for celiac disease should be included in strategies for investigating elevated transaminases. False positive tissue transglutaminase autoantibodies may occur in the setting of liver disease. However, a positive anti-endomysial antibody titer is quite specific even in this setting and maybe a preferable way to detect celiac disease in this regard.
Dermatological manifestations
Abenavoli et al. recently reviewed the dermatological diseases associated with CD, including dermatitis herpetiformis (DH), psoriasis, vitiligo, alopecia areata, Behcet's disease, oral lichen planus, dermatomyositis, pyoderma gangrenosum, and other more rare conditions. [44] DH is strongly associated with CD, tends to be more prevalent in males, and manifests as extremely pruritic, blistering lesions on the extensor surfaces of the extremities as well as back and buttocks; data from Finnish centers have shown that 1 in 4 CD patients are affected by DH. [45] A recent large, retrospective study by Alonso-Llamazares et al. found a 12.6% prevalence rate of CD and 22% prevalence rate of systemic autoimmune disorders among 263 DH patients. [46] Diagnosis is made by immunoflourescent visualization of granular IgA deposits at the junction of the epidermal/dermal layers in biopsies taken close to but not in an affected area. Epidermal tissue transglutaminase (eTg) is considered one of the key target autoantigens; the intestinal inflammation occurring in CD may be associated with increased production of antibodies directed against eTg. [47, 48] Lesions regress with oral Dapsone and compliance with a gluten free diet even in the absence of small bowel villous atrophy. [45] Recurrence of the lesions usually occurs with enteral or rectal challenge with gluten.
Ojetti et al. recently demonstrated a high prevalence of CD among 92 psoriasis patients (4.34%). Larger scale studies have not yet been reported. Michaelsson et al. showed improvement in psoriatic lesions following treatment with a gluten-free diet, most notably among patients with high anti-gliadin antibody titers and mild increases in duodenal epithelial lymphocytes or no enteropathy. [49] These studies raise interesting questions in terms of the immunological mechanisms connecting gastrointestinal and skin diseases and the opportunity for potential treatments. Multiple mechanisms have been proposed including, increased intestinal permeability, vitamin D deficiency, and gluten-induced T-cell activation. [50]
Neurological manifestations
Approximately 10 to 12% of celiac patients show neurological symptoms including, cerebellar ataxia, peripheral neuropathy, seizures, and myelopathy. [51] One small, retrospective study found a broad spectrum of these neurological disorders in 12 % of 148 pediatric and adult CD patients. [51] Cognitive decline, dementia, myopathy, and a rarer clinical condition involving epilepsy and cerebral calcifications in relation to CD have been described in case reports and series. [52-55]
Several recent studies have evaluated the prevalence and clinical presentation of ataxia and peripheral neuropathy associated with CD, as well as the potentially gluten-dependent neurological conditions occurring in the absence of enteropathy. Gluten ataxia is considered a distinct disease process which can occur with or without enteropathy and is associated with high anti-gliadin antibody titers. [56] Hadjivassiliou et al. demonstrated a 32% prevalence of anti-gliadin antibodies among patients with sporadic idiopathic ataxia. 24% of this gluten ataxia group had evidence of gluten-sensitive enteropathy and only 13% reported gastrointestinal symptoms. The mean age of neurological symptom onset was 48 years and the additional symptoms of dysarthria, upper and lower extremity ataxia, and ocular symptoms e.g. nystagmus were reported most frequently. [56] Anti-gliadin antibodies cross-reacting with epitopes on cerebellar Purkinje fibers is suggested by the authors as the likely mechanism. Whether gluten ataxia exists in the absence of celiac disease is controversial. Whilst antibodies to gliadin are common in patients with cerebellar ataxia, they are also common in other degenerative brain diseases such as Huntington's chorea and the genetically precipitated spinocerebellar ataxias.
Peripheral axonal neuropathy is a common neurological manifestation of CD. Prospective screening of 140 patients with idiopathic, axonal neuropathy demonstrated a CD prevalence of 9%. [57] Additionally in this study, among 100 patients with idiopathic neuropathy the mean age of neurological symptom onset was 55 years, 72% of patients had either DQ2 or DQ8 HLA typing, and significant variations in the types of neuropathy were reported. The authors propose the term “gluten neuropathy” to describe patients with idiopathic neuropathy and positive anti-gliadin antibody titers with or without evidence of enteropathy. Often, the autoimmune markers typifying CD are absent. Treatment with a gluten-free diet may improve neuropathic symptoms. [58, 59] However, it has not been definitively proven that gluten exclusion alters the natural history of this neurological state in the absence of true celiac disease.
Overall, multiple neurological manifestations of CD can occur. The more common manifestations include ataxia and peripheral neuropathy, while early onset dementia and cognitive decline may occasionally be due to celiac disease. The etiology of neurological syndromes related to gluten exposure though not directly associated with CD remains controversial.
IgA deficiency
The prevalence of CD among patients with IgA deficiency is greater than 10 fold higher as compared to controls. [60] Because IgA deficiency makes the detection of CD based on tissue transglutaminase (tTg) IgA titers unreliable, it is generally recommended to measure total levels of IgA to rule out a deficiency state. If IgA deficiency is present, tTG IgG is often, but not always, elevated prompting the need for small bowel biopsies and can be followed to measure occult gluten exposure or dietary indiscretion. Cataldo et al. found that patients with IgA deficiency had the silent form of CD more frequently and higher rates of allergic diseases including asthma, atopic dermatitis, and gastrointestinal allergies. [60]
Pernicious anemia
Dickey et al. showed a 12% prevalence of B12 deficiency among 159 celiac patients though no increased rates of pernicious anemia. [61] Assessments were based on gastric histology, intrinsic factor antibody testing, and serum gastrin levels. Only 2 of 19 patients displayed histological evidence of corpus atrophy. This is the largest study reported to date, demonstrating no increased prevalence of pernicious anemia. Though B12 deficiency is common it is likely due to other factors among CD patients.
Related GI Autoimmune Diseases
Refractory Celiac Disease
Some cases of CD do not respond to the removal of dietary gluten resulting in persistent villous atrophy, ongoing clinical symptoms, and increased risk for malignancy in the setting of continued immune activation. Refractory celiac disease (RCD) is a relatively rare complication among ∼2-5% of celiac patients. It is classified as persistent or recurrent symptoms of malabsorption and enteropathy. RCD is divided into two key categories, type I and type II. This distinction is important in terms of predicted morbidity and mortality, strategies for immunosuppressive therapy, and implications for enteropathy associated T-cell lymphoma (EATL) screening. The clinical and histologic presentation of these two groups bear similarities in the initial stages, specifically persistence of gastrointestinal symptoms, villous atrophy despite strict compliance with a gluten free diet for greater than 12 months, and normal tissue transglutaminase antibody titers. The distinction between type I and II RCD and its implications on overall patient survival are based on the monoclonal transformation of intraepithelial gamma, delta T-cell populations from the polyclonal phenotype characteristic of type I RCD. Diagnosis is made via immunohistochemical analysis of specific T-cell surface markers and PCR-based T-cell receptor gene rearrangement analysis. Referral to a specialized center for this testing and management are warranted.
Type II RCD increases the risk of developing ulcerative jejunitis and EATL. Capsule endoscopy is limited by the inability to obtain tissue biopsy, though double-balloon enteroscopy recently was found to be effective in diagnosing or excluding EATL +/- ulcerative jejunitis in patients with RCD. [62] One recent study found PET scanning to be of additional benefit in diagnosing early EATL and identifying extraluminal manifestations of lymphoma. [63] The authors suggest a combination PET-CT modality may offer even greater sensitivity for early detection. Additional risk factors associated with EATL include significant delay in diagnosis, DQ2 homozygous HLA status, and male sex. [64] Given the overall high mortality of EATL, early diagnosis and intervention are areas of current interest.
RCD I is generally steroid responsive and recommended first-line therapy is azathioprine following induction of clinical remission. Corticosteroid treatment is recommended for RCD II, though steroid resistant disease may warrant more aggressive immunosuppression regimens, including infliximab infusions. [65] Among RCD II patients, the risk of accelerating the development of a malignant T-cell clone with chronic immunosuppression makes this therapy controversial. Budesonide is effective in improving clinical symptoms in RCD I and II groups, though histological recovery of the small bowel architecture was not observed in one recent study. [66] Budesonide has significant first-pass metabolism, largely minimizing the systemic side effects associated with chronic steroid therapy.
Additional etiologies should be excluded prior to diagnosing RCD, including lymphocytic colitis, autoimmune enteropathy, pancreatic insufficiency, collagenous enterocolitis, bacterial overgrowth, and lactase deficiency. Screening and treatment for nutritional deficiencies including zinc, copper, B12, folate, iron indices, and albumin are generally recommended. In the event of severe hypoalbuminemia with associated edematous states and opportunistic infections, parenteral nutrition is frequently required.
Autoimmune Enteropathy (AIE)
Autoimmune enteropathy is a rare disease, predominantly occurring in children though cases of adult-onset have been reported. One of the initial reports of adult-onset autoimmune enteropathy was in 1997 from Corazza et al. describing two of four patients with DQ2 HLA typing, clinically non-responsive to gluten restriction, and who were found to be positive for anti-enterocyte antibodies. [67] Additional reported cases of adults with AIE or AIE variants include: AIE associated with atrophic gastritis and colitis, autoimmune enterocolitis, AIE in association with systemic autoimmune disease (sicca syndrome, thyroiditis), and AIE associated with rheumatoid arthritis. The pathophysiology of AIE is unclear. Leon et al. propose that TNF alpha production by activated T-cells in the bowel mucosa and a unique subset of CD4+ T-cells in the lamina propria may be important in the pathogenesis of disease.
Akram et al. recently published a case series describing the clinical and histological findings of 15 adults diagnosed with autoimmune enteropathy and proposed criteria for diagnosis. [68] This series included the frequency of associated autoimmune diseases and occurrence of autoimmune enterocolitis, a variant of AIE. This is the largest case series to date on this rare disorder. The cohort included patients with a median age of 55, predominantly Caucasian (87%), subtotal villous atrophy of the proximal small bowel, increased lymphocytosis within the lamina propria, positive anti-enterocyte antibody or anti-goblet cell antibody serology, and decreased number of intraepithelial lymphocytes. The clinical presentation included protracted diarrhea, weight loss, lack of responsiveness to a gluten restricted diet, and exclusion of additional secondary causes of villous atrophy.
Proposed criteria for diagnosis of adult AIE from the Mayo Clinic study includes: chronic diarrhea more than 6 weeks in duration, clinical signs and symptoms of malabsorption, specific histology on small bowel biopsy, exclusion of additional secondary diagnoses causing villous atrophy, positive anti-enterocyte or goblet cell antibody serologies; the first four criteria are necessary for diagnosis though negative serologies do not exclude the diagnosis of AIE. [68] Anti-enterocyte antibodies have not been reported in patients with inflammatory bowel disease or celiac disease. [68] 60% of patients in this study responded to high dose steroids, though 66% of this group became steroid dependent or required adjunct immunosuppressive therapy. 2 of the 15 patients were treated with infliximab with complete resolution of symptoms.
Given the rarity of AIE, specific associations with autoimmune disease would be difficult to establish. Systemic autoimmune diseases reported in the above study included: rheumatoid arthritis, hypothyroidism, psoriatic arthritis, autoimmune gastroenterocolitis, common variable immunodeficiency, myasthenia gravis, among others. One of the initial pediatric studies by Hill et al. (1991) reported varying degrees of colonic histopathologic changes in all 8 patients tested as compared to the controls. [69] Age of symptom onset varied from 2 to 12 months with a consistent interval of several months prior to diagnosis. Among the 8 patients and their first/second degree relatives, additional autoimmune diseases were present which included, hypothyroidism, interstitial nephropathy, chronic hepatitis, AIDDM, and vitiligo.
Collagenous Enteritis (Collagenous sprue)
Collagenous enteritis is closely related to celiac disease both histologically and clinically. Patients present with classic symptoms of malabsorption and histological evidence of villous atrophy on small bowel biopsy. Whether collagenous enteropathy is a variant of CD is controversial; features that distinguish this disorder from CD is the presence of a thickened, subepithelial collagen band and non-responsiveness to dietary gluten restriction. Positive anti-endomysial antibody serology and increased risk for T and B-cell lymphomas are additional shared features of both diseases. [70,71,72] The causes of collagenous enteritis are not known and treatment usually requires prolonged steroid therapy. Collagenous enteritis may occur in conjunction with collagenous colitis. [73, 74]
Microscopic colitis
Recent case reports and series have shown simultaneous occurrence of celiac disease and collagenous colitis. [75, 76] The series by Freeman showed 22.9% prevalence of CD among 35 patients with collagenous colitis. There is a clear association between CD and microscopic colitis, including both collagenous and lymphocytic colitis variants. Close to 30% of CD patients may have evidence of microscopic colitis on colon histology, while estimates range between 2 and 10% of microscopic colitis patients having villous atrophy suggestive of CD. [77] A low threshold for CD screening among these patient populations is warranted.
Summary
The strongest associations between CD and systemic autoimmune diseases include Addison's disease, autoimmune thyroid disease, AIDDM, Sjogren's syndrome, PBC, and autoimmune hepatitis (Figure 1). Though more cost-benefit analyses need to be done to support CD screening of these enriched populations and facilitate development of diagnostic algorithms, maintaining a low threshold for screening and high clinical suspicion among these groups is recommended. There is clear association between dermatitis herpetiformis (DH) and CD such that a definitive diagnosis of DH by direct immunofluorescence is sufficient evidence to justify initiation of a gluten free diet and positive tissue transglutaminase autoantibodies in the context of DH implies substantial villous atrophy. Testing among arthritic patients is less clear given the limited large scale studies, prevalence differences between adult and pediatric populations, and the varying types of arthritis studied to date. Maintaining a lower threshold for screening among pediatric as compared to adult populations is recommended. Screening for CD among SLE patients is not recommended, though should be considered in the differential diagnosis of lupus patients with gastrointestinal symptoms. Neurological patients with the broad spectrum of clinical symptoms described should be considered for CD screening and also evaluated for gluten-dependent conditions occurring in the absence of enteropathy. Additional autoimmune diseases of the small bowel should be considered in the absence of a clinical response to dietary gluten restriction and/or lack of the recommended diagnostic criteria for CD.
Figure 1.
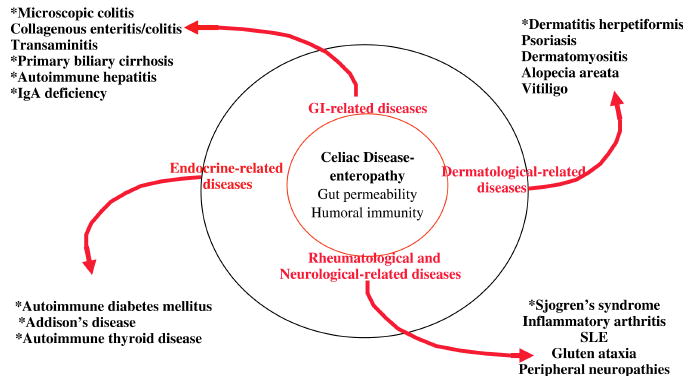
The connection between gut and systemic autoimmune diseases and the environmental factors affecting development is an evolving area of research. Further understanding of the underlying immune mechanisms will allow for the development of new therapies targeting intestinal permeability, mucosal regulatory T-cells, and neutralization of key inflammatory cytokines. The immunological basis for celiac disease provides a great opportunity to understand the interplay between genetic and environmental factors in autoimmune diseases in general.
Acknowledgments
Thanks to Dr. Eric V. Marietta for review of this manuscript.
Grant/funding Support: S.H.B. is supported by the NIH training grant T32 DK07198. J.A.M. is supported by NIH grants DK57892 and 071003.
Footnotes
Financial Disclosures: J.A.M. has been a consultant to Astra Zeneca, Alvine Inc., and Novartis and an investigator for Alba Therapeutics and Dynagen Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007 Jul;5(7):844–850. doi: 10.1016/j.cgh.2007.03.021. quiz 769. [DOI] [PubMed] [Google Scholar]
- 3.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002 Jan 17;346(3):180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 4.Cooper BT, Holmes GK, Cooke WT. Coeliac disease and immunological disorders. Br Med J. 1978 Mar 4;1(6112):537–539. doi: 10.1136/bmj.1.6112.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006 Sep;3(9):516–525. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 6.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. Jama. 2005 May 18;293(19):2343–2351. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 7.Ivarsson A. The Swedish epidemic of coeliac disease explored using an epidemiological approach--some lessons to be learnt. Best Pract Res Clin Gastroenterol. 2005 Jun;19(3):425–440. doi: 10.1016/j.bpg.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Ivarsson A, Hernell O, Nystrom L, Persson LA. Children born in the summer have increased risk for coeliac disease. J Epidemiol Community Health. 2003 Jan;57(1):36–39. doi: 10.1136/jech.57.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006 Oct;101(10):2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg G, Fahlgren A, Horstedt P, Hammarstrom S, Hernell O, Hammarstrom ML. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am J Gastroenterol. 2004 May;99(5):894–904. doi: 10.1111/j.1572-0241.2004.04157.x. [DOI] [PubMed] [Google Scholar]
- 11.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999 Aug;117(2):297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 12.Sategna Guidetti C, Solerio E, Scaglione N, Aimo G, Mengozzi G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut. 2001 Oct;49(4):502–505. doi: 10.1136/gut.49.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biagi F, Pezzimenti D, Campanella J, Corazza GR. Gluten exposure and risk of autoimmune disorders. Gut. 2002 Jul;51(1):140–141. doi: 10.1136/gut.51.1.140-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cataldo F, Marino V. Increased prevalence of autoimmune diseases in first-degree relatives of patients with celiac disease. J Pediatr Gastroenterol Nutr. 2003 Apr;36(4):470–473. doi: 10.1097/00005176-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Iltanen S, Collin P, Korpela M, et al. Celiac disease and markers of celiac disease latency in patients with primary Sjogren's syndrome. Am J Gastroenterol. 1999 Apr;94(4):1042–1046. doi: 10.1111/j.1572-0241.1999.01011.x. [DOI] [PubMed] [Google Scholar]
- 16.Szodoray P, Barta Z, Lakos G, Szakall S, Zeher M. Coeliac disease in Sjogren's syndrome--a study of 111 Hungarian patients. Rheumatol Int. 2004 Sep;24(5):278–282. doi: 10.1007/s00296-003-0360-x. [DOI] [PubMed] [Google Scholar]
- 17.Luft LM, Barr SG, Martin LO, Chan EK, Fritzler MJ. Autoantibodies to tissue transglutaminase in Sjogren's syndrome and related rheumatic diseases. J Rheumatol. 2003 Dec;30(12):2613–2619. [PubMed] [Google Scholar]
- 18.Stagi S, Giani T, Simonini G, Falcini F. Thyroid function, autoimmune thyroiditis and coeliac disease in juvenile idiopathic arthritis. Rheumatology (Oxford) 2005 Apr;44(4):517–520. doi: 10.1093/rheumatology/keh531. [DOI] [PubMed] [Google Scholar]
- 19.Lepore L, Martelossi S, Pennesi M, et al. Prevalence of celiac disease in patients with juvenile chronic arthritis. J Pediatr. 1996 Aug;129(2):311–313. doi: 10.1016/s0022-3476(96)70262-7. [DOI] [PubMed] [Google Scholar]
- 20.Francis J, Carty JE, Scott BB. The prevalence of coeliac disease in rheumatoid arthritis. Eur J Gastroenterol Hepatol. 2002 Dec;14(12):1355–1356. doi: 10.1097/00042737-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lubrano E, Ciacci C, Ames PR, Mazzacca G, Oriente P, Scarpa R. The arthritis of coeliac disease: prevalence and pattern in 200 adult patients. Br J Rheumatol. 1996 Dec;35(12):1314–1318. doi: 10.1093/rheumatology/35.12.1314. [DOI] [PubMed] [Google Scholar]
- 22.Bourne JT, Kumar P, Huskisson EC, Mageed R, Unsworth DJ, Wojtulewski JA. Arthritis and coeliac disease. Ann Rheum Dis. 1985 Sep;44(9):592–598. doi: 10.1136/ard.44.9.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza N, Bonilla E, Phillips PE. Celiac disease in a patient with systemic lupus erythematosus: a case report and review of literature. Clin Rheumatol. 2007 May;26(5):827–828. doi: 10.1007/s10067-006-0344-9. [DOI] [PubMed] [Google Scholar]
- 24.Rensch MJ, Szyjkowski R, Shaffer RT, et al. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am J Gastroenterol. 2001 Apr;96(4):1113–1115. doi: 10.1111/j.1572-0241.2001.03753.x. [DOI] [PubMed] [Google Scholar]
- 25.Courtney PA, Patterson RN, Lee RJ, McMillan SA. Systemic lupus erythematosus and coeliac disease. Lupus. 2004;13(3):214. doi: 10.1191/0961203304lu512xx. [DOI] [PubMed] [Google Scholar]
- 26.Richer O, Ulinski T, Lemelle I, et al. Abdominal manifestations in childhood-onset systemic lupus erythematosus. Ann Rheum Dis. 2007 Feb;66(2):174–178. doi: 10.1136/ard.2005.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallegua DS, Wallace DJ. Gastrointestinal manifestations of systemic lupus erythematosus. Curr Opin Rheumatol. 2000 Sep;12(5):379–385. doi: 10.1097/00002281-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sultan SM, Ioannou Y, Isenberg DA. A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology (Oxford) 1999 Oct;38(10):917–932. doi: 10.1093/rheumatology/38.10.917. [DOI] [PubMed] [Google Scholar]
- 29.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002 Jun;23(3):327–364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary C, Walsh CH, Wieneke P, et al. Coeliac disease and autoimmune Addison's disease: a clinical pitfall. Qjm. 2002 Feb;95(2):79–82. doi: 10.1093/qjmed/95.2.79. [DOI] [PubMed] [Google Scholar]
- 31.Betterle C, Lazzarotto F, Spadaccino AC, et al. Celiac disease in North Italian patients with autoimmune Addison's disease. Eur J Endocrinol. 2006 Feb;154(2):275–279. doi: 10.1530/eje.1.02089. [DOI] [PubMed] [Google Scholar]
- 32.Elfstrom P, Montgomery SM, Kampe O, Ekbom A, Ludvigsson JF. Risk of primary adrenal insufficiency in patients with celiac disease. J Clin Endocrinol Metab. 2007 Sep;92(9):3595–3598. doi: 10.1210/jc.2007-0960. [DOI] [PubMed] [Google Scholar]
- 33.Collin P, Kaukinen K, Valimaki M, Salmi J. Endocrinological disorders and celiac disease. Endocr Rev. 2002 Aug;23(4):464–483. doi: 10.1210/er.2001-0035. [DOI] [PubMed] [Google Scholar]
- 34.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Ch'ng CL, Jones MK, Kingham JG. Celiac disease and autoimmune thyroid disease. Clin Med Res. 2007 Sep;5(3):184–192. doi: 10.3121/cmr.2007.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatzicostas C, Roussomoustakaki M, Drygiannakis D, et al. Primary biliary cirrhosis and autoimmune cholangitis are not associated with coeliac disease in Crete. BMC Gastroenterol. 2002;2:5. doi: 10.1186/1471-230X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habior A, Lewartowska A, Orlowska J, et al. Association of coeliac disease with primary biliary cirrhosis in Poland. Eur J Gastroenterol Hepatol. 2003 Feb;15(2):159–164. doi: 10.1097/00042737-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007 Nov;46(5):1650–1658. doi: 10.1002/hep.21949. [DOI] [PubMed] [Google Scholar]
- 39.Ludvigsson JF, Elfstrom P, Broome U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol. 2007 Jan;5(1):63–69. e61. doi: 10.1016/j.cgh.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 40.Volta U, De Franceschi L, Molinaro N, et al. Frequency and significance of anti-gliadin and anti-endomysial antibodies in autoimmune hepatitis. Dig Dis Sci. 1998 Oct;43(10):2190–2195. doi: 10.1023/a:1026650118759. [DOI] [PubMed] [Google Scholar]
- 41.Villalta D, Girolami D, Bidoli E, et al. High prevalence of celiac disease in autoimmune hepatitis detected by anti-tissue tranglutaminase autoantibodies. J Clin Lab Anal. 2005;19(1):6–10. doi: 10.1002/jcla.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czaja AJ. Autoimmune Liver Disease. In: Zakim D, B T, editors. Hepatology: a textbook of liver disease. Vol. 2. Philadelphia: Saunders; 2003. pp. 1163–1202. [Google Scholar]
- 43.Bridoux-Henno L, Dabadie A, Briard D, Bahon-Riedinger I, Jouan H, Le Gall E. A case of celiac disease presenting with autoimmune hepatitis and erythroblastopenia. J Pediatr Gastroenterol Nutr. 2001 Nov;33(5):616–619. doi: 10.1097/00005176-200111000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Abenavoli L, Proietti I, Leggio L, et al. Cutaneous manifestations in celiac disease. World J Gastroenterol. 2006 Feb 14;12(6):843–852. doi: 10.3748/wjg.v12.i6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collin P, Reunala T. Recognition and management of the cutaneous manifestations of celiac disease: a guide for dermatologists. Am J Clin Dermatol. 2003;4(1):13–20. doi: 10.2165/00128071-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 46.Alonso-Llamazares J, Gibson LE, Rogers RS., 3rd Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. Int J Dermatol. 2007 Sep;46(9):910–919. doi: 10.1111/j.1365-4632.2007.03214.x. [DOI] [PubMed] [Google Scholar]
- 47.Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002 Mar 18;195(6):747–757. doi: 10.1084/jem.20011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marietta EV, Camilleri MJ, Castro LA, Krause PK, Pittelkow MR, Murray JA. Transglutaminase Autoantibodies in Dermatitis Herpetiformis and Celiac Sprue. J Invest Dermatol. 2007 Aug 30; doi: 10.1038/sj.jid.5701041. [DOI] [PubMed] [Google Scholar]
- 49.Michaelsson G, Gerden B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000 Jan;142(1):44–51. doi: 10.1046/j.1365-2133.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 50.Abenavoli L, Leggio L, Gasbarrini G, Addolorato G. Celiac disease and skin: psoriasis association. World J Gastroenterol. 2007 Apr 14;13(14):2138–2139. doi: 10.3748/wjg.v13.i14.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaknin A, Eliakim R, Ackerman Z, Steiner I. Neurological abnormalities associated with celiac disease. J Neurol. 2004 Nov;251(11):1393–1397. doi: 10.1007/s00415-004-0550-9. [DOI] [PubMed] [Google Scholar]
- 52.Hu WT, Murray JA, Greenaway MC, Parisi JE, Josephs KA. Cognitive impairment and celiac disease. Arch Neurol. 2006 Oct;63(10):1440–1446. doi: 10.1001/archneur.63.10.1440. [DOI] [PubMed] [Google Scholar]
- 53.Collin P, Pirttila T, Nurmikko T, Somer H, Erila T, Keyrilainen O. Celiac disease, brain atrophy, and dementia. Neurology. 1991 Mar;41(3):372–375. doi: 10.1212/wnl.41.3.372. [DOI] [PubMed] [Google Scholar]
- 54.Gobbi G. Coeliac disease, epilepsy and cerebral calcifications. Brain Dev. 2005 Apr;27(3):189–200. doi: 10.1016/j.braindev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Hadjivassiliou M, Chattopadhyay AK, Grunewald RA, et al. Myopathy associated with gluten sensitivity. Muscle Nerve. 2007 Apr;35(4):443–450. doi: 10.1002/mus.20709. [DOI] [PubMed] [Google Scholar]
- 56.Hadjivassiliou M, Grunewald R, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003 Mar;126(Pt 3):685–691. doi: 10.1093/brain/awg050. [DOI] [PubMed] [Google Scholar]
- 57.Hadjivassiliou M, Grunewald RA, Kandler RH, et al. Neuropathy associated with gluten sensitivity. J Neurol Neurosurg Psychiatry. 2006 Nov;77(11):1262–1266. doi: 10.1136/jnnp.2006.093534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rigamonti A, Magi S, Venturini E, Morandi L, Ciano C, Lauria G. Celiac disease presenting with motor neuropathy: effect of gluten free-diet. Muscle Nerve. 2007 May;35(5):675–677. doi: 10.1002/mus.20727. [DOI] [PubMed] [Google Scholar]
- 59.Hadjivassiliou M, Kandler RH, Chattopadhyay AK, et al. Dietary treatment of gluten neuropathy. Muscle Nerve. 2006 Dec;34(6):762–766. doi: 10.1002/mus.20642. [DOI] [PubMed] [Google Scholar]
- 60.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut. 1998 Mar;42(3):362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002 Apr;14(4):425–427. doi: 10.1097/00042737-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Hadithi M, Mallant M, Oudejans J, van Waesberghe JH, Mulder CJ, Comans EF. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. J Nucl Med. 2006 Oct;47(10):1622–1627. [PubMed] [Google Scholar]
- 63.Hadithi M, Al-toma A, Oudejans J, van Bodegraven AA, Mulder CJ, Jacobs M. The value of double-balloon enteroscopy in patients with refractory celiac disease. Am J Gastroenterol. 2007 May;102(5):987–996. doi: 10.1111/j.1572-0241.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- 64.Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007 Oct;56(10):1373–1378. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005 Jun;19(3):413–424. doi: 10.1016/j.bpg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Brar P, Lee S, Lewis S, Egbuna I, Bhagat G, Green PH. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007 Oct;102(10):2265–2269. doi: 10.1111/j.1572-0241.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 67.Corazza GR, Biagi F, Volta U, Andreani ML, De Franceschi L, Gasbarrini G. Autoimmune enteropathy and villous atrophy in adults. Lancet. 1997 Jul 12;350(9071):106–109. doi: 10.1016/S0140-6736(97)01042-8. [DOI] [PubMed] [Google Scholar]
- 68.Akram S, Murray JA, Pardi DS, et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007 Nov;5(11):1282–1290. doi: 10.1016/j.cgh.2007.05.013. quiz 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill SM, Milla PJ, Bottazzo GF, Mirakian R. Autoimmune enteropathy and colitis: is there a generalised autoimmune gut disorder? Gut. 1991 Jan;32(1):36–42. doi: 10.1136/gut.32.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freeman HJ. Hyposplenism, antiendomysial antibodies and lymphocytic colitis in collagenous sprue. Can J Gastroenterol. 1999 May;13(4):347–350. doi: 10.1155/1999/596427. [DOI] [PubMed] [Google Scholar]
- 71.Freeman HJ. Collagenous mucosal inflammatory diseases of the gastrointestinal tract. Gastroenterology. 2005 Jul;129(1):338–350. doi: 10.1053/j.gastro.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 72.Freeman HJ. Collagenous sprue associated with an extensive T-cell lymphoma. J Clin Gastroenterol. 2003 Feb;36(2):144–146. doi: 10.1097/00004836-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Schreiber FS, Eidt S, Hidding M, Schmidt-Walczuch J, Werning C. Collagenous duodenitis and collagenous colitis: a short clinical course as evidenced by sequential endoscopic and histologic findings. Endoscopy. 2001 Jun;33(6):555. doi: 10.1055/s-2001-14968. [DOI] [PubMed] [Google Scholar]
- 74.McCashland TM, Donovan JP, Strobach RS, Linder J, Quigley EM. Collagenous enterocolitis: a manifestation of gluten-sensitive enteropathy. J Clin Gastroenterol. 1992 Jul;15(1):45–51. [PubMed] [Google Scholar]
- 75.Freeman HJ. Collagenous colitis as the presenting feature of biopsy-defined celiac disease. J Clin Gastroenterol. 2004 Sep;38(8):664–668. doi: 10.1097/01.mcg.0000135363.12794.2b. [DOI] [PubMed] [Google Scholar]
- 76.Smith P, Bishop P, Whorwell PJ. Collagenous colitis, ulcerative colitis, coeliac disease and hyperparathyroidism in one patient: implications for the management of collagenous colitis. Eur J Gastroenterol Hepatol. 2005 Nov;17(11):1239–1242. doi: 10.1097/00042737-200511000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Pardi DS. Microscopic colitis. Mayo Clin Proc. 2003 May;78(5):614–616. doi: 10.4065/78.5.614. quiz 616-617. [DOI] [PubMed] [Google Scholar]
- 78.Myhre AG, Aarsetoy H, Undlien DE, Hovdenak N, Aksnes L, Husebye ES. High frequency of coeliac disease among patients with autoimmune adrenocortical failure. Scand J Gastroenterol. 2003 May;38(5):511–515. doi: 10.1080/00365520310002544. [DOI] [PubMed] [Google Scholar]
- 79.Biagi F, Campanella J, Soriani A, Vailati A, Corazza GR. Prevalence of coeliac disease in Italian patients affected by Addison's disease. Scand J Gastroenterol. 2006 Mar;41(3):302–305. doi: 10.1080/00365520500206517. [DOI] [PubMed] [Google Scholar]


