Abstract
Alpha tocopherol transfer protein (ATTP) null mice (ATTP−/−) have a systemic deficiency of alpha-tocopherol (AT). The heart AT levels of ATTP−/− are <10% of those in ATTP+/+ mice. The genomic responses of heart to AT deficiency were determined in 3 months old male ATTP−/− mice and compared with their ATTP+/+ littermate controls using Affymetrix 430A 2.0 high density oligonucleotide arrays. Differential analysis of ~13,000 genes identified repression of genes related to immune system and activation of genes related to lipid metabolism and inflammation with no significant change in the expression of classical antioxidant genes (catalase, superoxide dismutase, glutathione peroxidase) in ATTP−/− as compared to ATTP+/+ mice. The present data identifies novel classes of AT sensitive genes in heart tissue.
Keywords: alpha-tocopherol, alpha-tocopherol transfer protein null mice, heart, gene expression profiling
1. Introduction
Vitamin E (VE) was first discovered as a “reproductive factor” important in the maintenance of pregnancy in rats [1]. α-tocopherol (AT), a lipid-soluble antioxidant, is the most abundant biologically active form of VE [2]. VE is absorbed from the intestine, initially transported to systemic tissues in chylomicrons, then following uptake of chylomicrons and their remnants by the liver, with >90% of the absorbed tocopherols still intact, incorporated into the secreted very low density lipoproteins, resulting in recirculation of VE within lipoprotein compartments [3]. AT is preferentially incorporated into the secreted lipoproteins due to its high affinity of AT for transfer protein (ATTP), a protein found predominantly in the liver [4]. The biological activity of AT is thus dependent upon its delivery to tissues, and decreases in the binding capacity or affinity of ATTP for AT will limit the secretion of AT into lipoproteins and the subsequent delivery to peripheral tissues. In the ATTP−/− mice, low AT concentrations are present in plasma (approx. 5% of ATTP+/+) and in extra-hepatic tissues, including heart (2–20%), but liver concentrations remain approximately 40% of those of ATTP+/+ mice [5].
Atherosclerotic cardiovascular diseases, a major cause of death in the United States, are believed to be related in part to lipid peroxidative processes occurring in blood vessel walls. As AT represents the major biologic lipophilic antioxidant, many clinical trials have tested the efficacy of AT alone or in combination with other antioxidants to ameliorate cardiovascular diseases, but with results that are generally negative and at best far from clear [6,7]. Little has been reported concerning heart tissue genomic responses to AT. The present study was carried out to identify global gene expression changes in heart tissue as a result of AT deficiency secondary to deletion of the ATTP gene.
2. Materials and Methods
2.1 Animals and Diet
The protocols for the care and use of animals were approved by the Institutional Care and Use Committee at the University of California, Davis. Male C57BL/6 mice with a deletion of ATTP gene (ATTP−/−) and littermate wild type mice (ATTP+/+) were used from our colonies, which originated from that described by Terasawa et al [8]. The mice were housed in polycarbonate cages in a room maintained at 21–23 °C and 60–70% humidity on a 12 h light/dark schedule and with ad libitum access to water and food. The offsprings were genotyped as previously described [8]. After weaning, the offsprings were fed diets containing 35 IU dl-tocopheryl acetate per kg diet (USB Corporation, Cleveland, OH). At 12–14 weeks the animals were sacrificed by i.p. injection of beuthanasia (120 mg/kg body weight), blood was obtained by cardiac puncture and tissue samples were obtained and stored at −80°C until further processing.
2.2 RNA Extraction, Gene Chip Analysis & Statistics
Total RNA from heart tissue was extracted and processed for GeneChip analysis. RNA from heart tissues were extracted with Trizol reagent and purified and quantified according to the manufacturer’s (Invitrogen) protocol. An equal aliquot (5 μg) of total RNA extract in RNAase- and DNAase-free water from each tissue from the group of mice (n = 4) was combined. An aliquot (20 μg) of pooled RNA solution was used for preparation of biotinlabeled RNA for hybridization using Affymetrix Mouse 430A 2.0 arrays containing oligonucleotide probes for ~22,690 genes (Santa Clara, CA, USA). The scanned images of hybridization signals were analyzed with the Affymetrix GeneChip Operating Software (GCOS 1.4) and Data Mining Tool software. The absolute mRNA expression (present or absent) and differential (ATTP+/+ vs ATTP−/−) mRNA expression data were obtained from the pivot data. GeneChips contain 11 pairs of probes to obtain specific and non-specific binding. Net binding intensities for each mRNA were computed by GCOS 1.4, each pair of probes resulting in a total of 11 intensities for each mRNA. Mean, standard deviation and p values are calculated from these data. When the P value for detection signal was <0.049 (range of P value 0.0002–0.049), the expression of the mRNA was classified as present (P). All mRNAs with the p value for detection >0.05 were considered absent (A). Genes whose expression changed by ≥2-fold were considered for further analyses. We also performed Gene Ontology (GO) analysis to assess the content of differentially expressed genes for characterizing the biological properties and generated heat-map by dChip software [9].
3. Results
Though the direct action of AT on heart tissue is not clear, early studies suggested a relationship of nutritional VE deficiency to cardiomyopathy [10,11] and postulated it to be the consequences of oxidative stress [12]. In the present study, we analyzed the global gene expression profile of heart tissue of ATTP−/− mice which have systemic deficiency of AT. The microarray analysis by Affymetrix gene chips detected ~13192 genes out of which 65 genes were affected in heart tissue of ATTP−/− mice as compared to that of ATTP+/+ mice. Of these 65 genes, 34 were upregulated (30 with known functions; 4 unknown functions) and 31 were downregulated (28 with known functions; 3 unknown functions). The differentially expressed genes were classified by GO ontology (www.geneontology.org) using dChip software. Genes related to immune responses (19%) were seen to be largely affected by AT deficiency and genes related to the regulation of cellular physiological processes and protein modification (10% and 8% respectively) were also seen to be affected [Fig 1]. The heat-map of the differentially expressed genes with known functions generated by dChip software is reported in Fig 2. Downregulated and Upregulated genes listed according to molecular function are reported in Tables 1 and 2, respectively.
Fig 1.
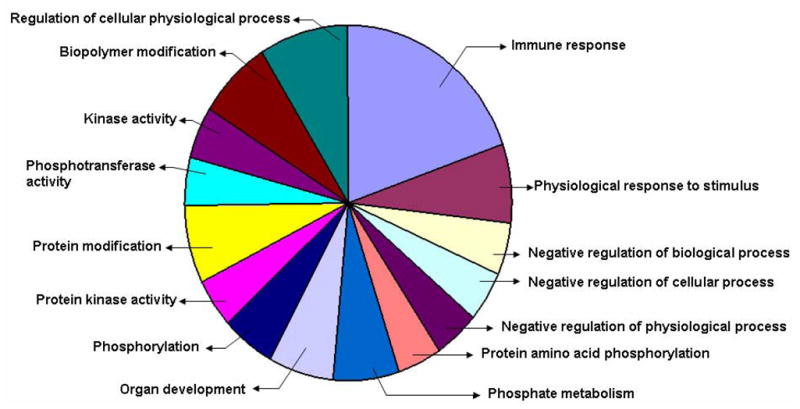
Functional classification of differentially expressed heart genes in ATTP−/− mice as compared to ATTP+/+ mice.
The list of differentially expressed genes was obtained with GCOS 1.4 software. The list was then edited to select genes of known function that change by ≥ 2-fold. The edited list of genes was subjected to GO ontology software and classified according to biological functions. Note that same genes might be included in several functional groups as per its biological function.
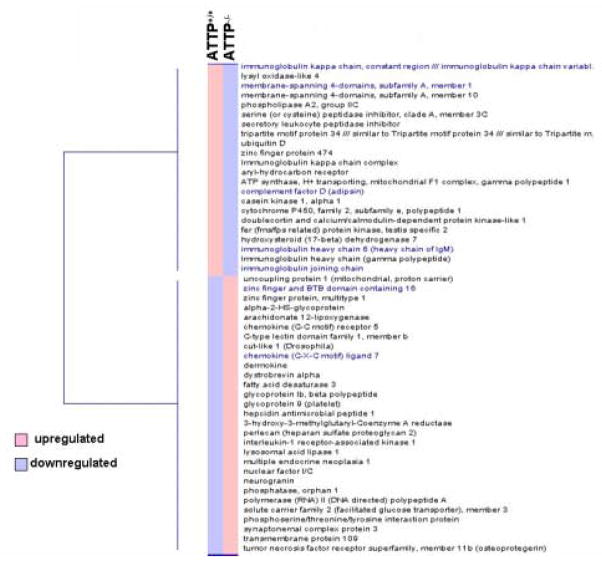
Fig 2.
Heat-map of identified differentially expressed heart genes in ATTP−/− mice as compared to ATTP+/+ mice generated by d-Chip software.
The list of differentially expressed, AT-sensitive genes was obtained with GCOS software. The list was further edited to delete genes of unknown function and to focus on genes that change by ≥ 2-fold. The edited list of AT-sensitive genes was subjected to “hierarchial cluster analysis” with d-Chip software. The analysis identified two major clusters. The “two-colour” heat map shows downregulated (blue) and upregulated (pink) genes.
Table 1.
Downregulated genes in heart tissue of ATTP−/− mice as compared to ATTP+/+ mice
| Probe Set ID | Gene Title | Gene Symbol | fold change |
|---|---|---|---|
| Immune Response | |||
| 1452417_x_at | immunoglobulin kappa chain variable 8 (V8)–16 | Igk-V8–16 | −19.7 |
| 1424305_at | immunoglobulin joining chain | Igj | −14.9 |
| 1424631_a_at | Immunoglobulin heavy chain (gamma polypeptide) | Ighg | −13.0 |
| 1452463_x_at | Immunoglobulin kappa chain complex | -- | −12.1 |
| 1427329_a_at | immunoglobulin heavy chain 6 (heavy chain of IgM) | Igh-6 | −7.0 |
| 1419762_at | ubiquitin D | Ubd | −9.2 |
| 1448377_at | secretory leukocyte peptidase inhibitor | Slpi | −2.1 |
| 1417867_at | complement factor D (adipsin) | Cfd | −2.6 |
| 1421564_at | serine (or cysteine) peptidase inhibitor, clade A, member 3C | Serpina3c | −2.3 |
|
Signal Transduction | |||
| 1451497_at | casein kinase 1, alpha 1 | Csnk1a1 | −26.0 |
| 1432453_a_at | membrane-spanning 4-domains, subfamily A, member 10 | Ms4a10 | −10.6 |
| 1450863_a_at | doublecortin and calcium/calmodulin-dependent protein kinase-like 1 | Dcamkl1 | −4.0 |
| 1426090_a_at | fer (fms/fps related) protein kinase, testis specific 2 | Fert2 | −3.7 |
| 1423226_at | membrane-spanning 4-domains, subfamily A, member 1 | Ms4a1 | −2.6 |
|
Extracellular Matrix Protein | |||
| 1450134_at | lysyl oxidase-like 4 | Loxl4 | −5.3 |
|
Lipid Metabolism | |||
| 1417871_at | hydroxysteroid (17-beta) dehydrogenase 7 | Hsd17b7 | −4.6 |
| 1420584_at | phospholipase A2, group IIC | Pla2g2c | −3.5 |
|
Transcription factors | |||
| 1450695_at | aryl-hydrocarbon receptor | Ahr | −4.0 |
| 1451059_at | zinc finger protein 474 | Zfp474 | −2.8 |
| 1421550_a_at | tripartite motif protein 34 | Trim34 | −2.1 |
|
Xenobiotic Metabolism | |||
| 1415994_at | cytochrome P450, family 2, subfamily e, polypeptide 1 | Cyp2e1 | −2.1 |
|
Ion Transport | |||
| 1438809_at | ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1 | Atp5c1 | −2.0 |
Table 2.
Upregulated genes in heart tissue of ATTP−/− mice as compared to ATTP+/+ mice
| Probe Set ID | Gene Name | Gene Symbol | Fold change |
|---|---|---|---|
|
Lipid Metabolism | |||
| 1427229_at | 3-hydroxy-3-methylglutaryl-Coenzyme A reductase | Hmgcr | 8.0 |
| 1423140_at | Lysosomal acid lipase 1 | Lip1 | 2.6 |
| 1422699_at | Arachidonate 12-lipoxygenase | Alox12 | 2.5 |
| 1449219_at | Fatty acid desaturase 3 | Fads3 | 2.5 |
| 1418197_at | Uncoupling protein 1 (mitochondrial, proton carrier) | Ucp1 | 3.2 |
|
Inflammatory Response | |||
| 1919720_at | Glycoprotein 9 | Gp9 | 8.6 |
| 1449033_at | Tumor necrosis factor receptor superfamily, member 11b | Tnfrsf11b | 4.6 |
| 1422977_at | Glycoprotein 1b, beta polypeptide | Gp1bb | 2.8 |
| 1419196_at | Hepcidin antimicrobial peptide 1 | Hamp1 | 2.5 |
| 1421182_at | C-type lectin domain family 1, member b | Clec1b | 2.3 |
| 1424727_at | Chemikine (C-C motif) receptor 5 | Ccr5 | 2.1 |
| 1418480_at | Chemokine (C-X-C motif) ligand 7 | Cxcl7 | 2.1 |
| 1455093_a_at | Alpha-2-HS-glycoprotein | Ahsg | 2.1 |
| 1460649_at | Interleukin-1 receptor-associated kinase 1 | Irak1 | 2.0 |
| 1418669_at | Perlecan (heparin sulfate proteoglycan 2) | Hspg2 | 2.0 |
|
Cytoskeletal function | |||
| 1456069_at | Dystrobrevin alpha | Dtna | 4.6 |
| 1428781_at | Dermokine | Dmkn | 2.6 |
|
Binding Protein | |||
| 1423231_at | neurogranin | Nrgn | 3.5 |
|
Transporter | |||
| 1455898_x_at | Solute carrier family 2 (facilitated glucose transporter), member 3 | Slc2a3 | 3.2 |
|
Transcription factors | |||
| 1422565_s_at | Nuclear factor I/C | Nfic | 2.3 |
| 1419874_x_at | Zinc finger and BTB domain containing 16 | Zbtb16 | 2.3 |
| 1416348_at | Multiple endocrine neoplasia 1 | Men1 | 2.1 |
| 1449534_at | Synaptonemal complex protein 3 | Sycp3 | 2.1 |
| 1423603_at | Zinc finger protein, multitype 1 | Zfpm1 | 2.1 |
| 1424667_a_at | Cut-like 1 (Drosophila) | Cutl1 | 2.0 |
| 1426242_at | Polymerase (RNA) II (DNA directed) polypeptide A | Polr2a | 2.0 |
|
Other functions | |||
| 1416032_at | Transmembrane protein 109 | Tmem109 | 2.6 |
| 1421664_a_at | Phosphoserine/threonine/tyrosine interaction protein | Styx | 2.3 |
| 1452485_at | Phosphatase, orphan 1 | Phospho1 | 2.0 |
Of note, a cluster of genes related to immune functions (Igk-V8–16, Igj, Ighg, Igh-6, Ubd, Slpi, Cfd) were downregulated (Table 1) whereas genes related to lipid metabolism (Hmgcr, Lip1, Alox12, Fads3, Ucp1) and inflammatory response (Gp9, Tnfrsf11b, Gp1bb, Hamp1, Clec1b, Ccr5, Cxcl7, Irak1) were upregulated (Table 2).
4. Discussion
The present study was carried out to analyze the global gene expression profiling of heart tissue of ATTP−/− mice deficient in AT. We have previously reported low heart AT levels (1.97 ± 0.52 nmol/g wet weight tissue) in ATTP−/− mice as compared to their respective ATTP+/+ controls (16.02 ± 4.46 nmol/g wet weight tissue) fed a basal diet [13], heart AT levels in ATTP−/− mice thus being approximately 10% of levels of their ATTP+/+ littermates. AT levels from human myocardial biopsies have been reported to be 61 ± 4 nmol/g wet weight tissue [14], which is approximately 4 times higher than the myocardial levels found in the ATTP+/+ mice [13]. The plasma AT levels of ATTP+/+ mice, ATTP−/− mice and humans are reportedly ~ 4.5 μmol/l, 0.5 μmol/l [13] and 17 μmol/l [15], respectively, and suggest that myocardial AT levels are related to plasma AT levels.
ATTP gene expression was not detected in heart tissues of ATTP+/+ mice, confirming our earlier reported data on heart ATTP mRNA by RT-PCR analysis in ATTP+/+ and ATTP−/− mice [15] and in human heart tissue by Arita et al [16]. Hence the drop in heart AT levels in ATTP−/− mice is likely attributed to lower plasma AT concentrations [5]. Though AT deficiency is suggested to cause oxidant-antioxidant imbalance leading to oxidative stress [12], in the present study no significant changes were detected in the classical antioxidant genes such as catalase, superoxide dismutase or glutathione peroxidase in heart tissues of ATTP−/− mice as compared to their ATTP+/+ littermates. We have recently reported similar observations in lung tissues of ATTP+/+ mice fed an AT deficient diet [17] and in lungs of ATTP−/− mice [18].
Several antioxidant clinical trails testing the efficacy of AT on cardiovascular and related diseases were inconclusive [6,7]. This and other considerations have prompted researchers to explore the modulation of biological systems by AT beyond its “non-antioxidant” properties. In 1988, Mahoney and Azzi, for the first time, reported the inhibitory effect of AT on brain protein kinase C (PKC) activity in vitro [19]. AT-induced PKC inhibition was also reported to inhibit smooth muscle cell proliferation and platelet adhesion, aggregation and release reactions, both believed to be independent of AT antioxidant properties [20–23]. AT also inhibited native and oxidized low density lipoprotein (LDL)-induced PKC activity and proliferation of vascular smooth muscle cells, thus affecting processes related to atherogenesis [24]. Of note, AT itself has not been noted to be deficient in human atherosclerotic plaques in spite of the presence of co-existing oxidized lipids [25]. One possible explanation is that the oxidation of lipoproteins by two electron reactions, such as by hypochlorite and peroxynitrite, is not known to be quenched by AT [26,27].
Meydani and co-workers have reported that AT is essential for maintaining optimal immune system functions [28] and that AT deficiency dysregulates immune responses and increase susceptibilities to various infections [29,30]. Supplementation of AT to old mice infected by influenza virus was reported to improve immune response by increasing T helper 1 (Th1) cytokines [31] and by decreasing the age-associated decline in CD(+) T cells signalling [32]. In the present study, a cluster of genes related to immune function was seen to be downregulated in heart tissues of ATTP−/− mice (Table 1). The immunoglobulin related genes, such as immunoglobulin kappa chain variable 8 (V8)–16 (Igk-V8–16), immunoglobulin joining chain (IgJ), immunoglobulin heavy chain (gamma polypeptide) (Ighg) and immunoglobulin heavy chain 6 (heavy chain of IgM) (Igh-6) were downregulated by −19.7, −14.9, −13 and −7 fold, respectively (Table 1). We had previously reported similar observations in lung genomic profiles of ATTP−/− mice [18]. AT deficiency was also reported to intensify viral infections and myocardial injury in mice fed AT-deficient diets [33].
Complement factor D (Cfd; or adipsin), a key component in the activation of the alternate pathway innate immunity [34], was downregulated by −2.6 fold. Cfd, secreted from cells of nervous tissue and adipocytes, has been implicated to regulate fat metabolism [35]. Secretory leukocyte peptidase inhibitor (Slpi) which protects against microbial infection and subsequent inflammation [36], was also downregulated by −2.1 fold. Ubiquitin D (Ubd; or FAT10) was downregulated by −9.2 fold. Of note, the lymphocytes of Ubd deficient mice were seen to be more prone to spontaneous apoptotic death and demonstrated high sensitivity to endotoxin challenge [37]. Casein kinase 1 (Csnk1a1), a serine/threonine protein kinase which phosphorylates a variety of substrates [38], was seen to be downregulated by −26 fold. This is particularly interesting as casein kinase 1 is known as a positive regulator of Wnt pathway [39], and the interruption of this pathway in heart might lead to impaired tissue remodeling [40].
The upregulation of mRNA encoding 3-hydroxy-3-methylglutaryl-Coenzyme A reductase (Hmgcr), the key enzyme for cholesterol biosynthesis, lysosomal acid lipase 1 (Lip1), fatty acid desaturase 3 (Fads3) [41,42] by 8.0, 2.6 and 2.5 fold, respectively, suggests dysregulated lipid metabolism in hearts of ATTP−/− mice. The upregulation of lipid homeostasis related genes were also observed in lungs of ATTP−/− mice (18). Uncoupling protein 1 (Ucp-1), a mediator of proton leakage in mitochondria and believed to be involved in thermogenesis and energy expenditure [43], and suggested to play a role in the transport of fatty acids across mitochondrial membrane [44], was upregulated by 3.2 fold (Table 2).
Genes encoding glycoprotein 9 (Gp9) and glycoprotein Ib, beta polypeptide (Gp1bb), factors relating to platelet activation and aggregation [45], were upregulated by 8.6 and 2.8 fold, respectively (AT supplementation was reported to inhibit platelet aggregation [21]). Tumor necrosis factor receptor superfamily, member 11b (Tnfrsf11b; osteoprotegerin), which was upregulated by 4.6 fold, was recently reported to be elevated in patients with atherosclerotic cardiovascular disease [46]. The above mentioned genes, as well as the upregulation of other genes related to inflammatory processes such as those encoding arachidonate 12-lipoxygenase (Alox12) [47], chemokine (C-C motif) receptor 5 (Ccr5) [48], chemokine (C-X-C motif) ligand 7 (Ccl7) [49] and interleukin-1 receptor-associated kinase 1 (Irak-1) [50], suggest increased inflammatory responses in ATTP−/− cardiac tissues as a result of AT deficiency, as observed in our earlier reports in ATTP−/− lung and liver tissues [51]. Expression of alpha 2-HS-glycoprotein (Ahsg), which shows negative acute-phase reactant properties, was upregulated by 2.1 fold and has been identified in the mineralized matrix of calcified plaques of atherosclerotic human aortas [52]. It is interesting to note that cytochrome P450 -2E1 (Cyp2e1), which has a capability of initiating lipid peroxidation by generation of reactiveoxygen species [53], was downregulated by −2.1 fold.
Patients with 744 del A mutation on ATTP gene were reported to show cardiomyopathy as evidenced by echocardiography, but this was not observed in all patients with this disorder [54]. In the present studies we have not investigated for evidences of cardiomyopathy in ATTP−/− mice. Further studies are warranted in these regard.
In summary, we report a preliminary characterization of the genomic profile in heart tissues of AT deficient mice occurring as a result of ATTP gene deletion. The data suggest dysregulation in lipid metabolism and immune-related functions in ATTP−/− mice. The implications of the current observations to human cardiomyopathies associated with activation of inflammatory processes and oxidative stress remain to be characterized. The present microarray data failed to detect modifications of classical antioxidant genes (catalase, superoxide dismutases or glutathione peroxidases), further suggesting that non-antioxidant properties of AT are likely responsible for the presently archived modulations in cardiac gene expression (55,56).
Acknowledgments
Research was supported in part by grants from National Institute of Health Sciences [NIEHS ES 011985] and United States Department of Agriculture [USDA NRICGP 2003–00915].
Abbreviations
- Ahsg
alpha 2-HS-glycoprotein
- Alox12
arachidonate 12-lipoxygenase
- AT
alpha-tocopherol
- ATTP
alpha-tocopherol transfer protein
- Ccr5
chemokine (C-C motif) receptor 5
- Cfd
complement factor D
- Clec1b
C-type lectin domain family 1, member b
- Csnk1a1
casein kinase 1
- Cxcl7
chemokine (C-X-C motif) ligand 7
- Cyp2e1
cytochrome P450, family 2, subfamily e, polypeptide 1
- Fads3
fatty acid desaturase 3
- GCOS
gene chip operating software
- Gp1bb
glycoprotein 1b, beta polypeptide
- Gp9
glycoprotein 9
- Hamp1
hepcidin antimicrobial peptide 1
- Hmgcr
3-hydroxy-3-methylglutaryl-Coenzyme A reductase
- Igh-6
immunoglobulin heavy chain 6 (heavy chain of IgM)
- Ighg
immunoglobulin heavy chain (gamma polypeptide)
- Igk-V8–16
immunoglobulin kappa chain variable 8 (V8)–16
- Irak1
interleukin-1 receptor-associated kinase 1
- Lip1
lysosomal acid lipase 1
- PKC
protein kinase C
- Slpi
secretory leukocyte peptidase inhibitor
- Tnfrsf11b
tumor necrosis factor receptor superfamily, member 11b
- Ubd
ubiquitin D
- Ucp1
uncoupling protein 1
- VE
vitamin E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C. Chemistry and biology of Vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 3.Traber MG, Arai H. Molecular mechanisms of Vitamin E transport. Annu Rev Nutr. 1999;19:343–355. doi: 10.1146/annurev.nutr.19.1.343. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y, Hagiwara K, Arai H, Inoue K. Purification and characterization of the α-tocopherol transfer protein from rat liver. FEBS Lett. 1991;288:41–45. doi: 10.1016/0014-5793(91)80999-j. [DOI] [PubMed] [Google Scholar]
- 5.Leonard SW, Terasawa Y, Farese RV, Jr, Traber MG. Incorporation of deuterated RRR- or all-rac-α-tocopherol in plasma and tissues of α-tocopherol transfer protein-null mice. Am J Clin Nutr. 2002;75:555–560. doi: 10.1093/ajcn/75.3.555. [DOI] [PubMed] [Google Scholar]
- 6.Jialal I, Devaraj S. Scientific evidence to support a Vitamin E and Heart Disease Health Claim: Research Needs. Am J Clin Nutr. 2005;135:348–353. doi: 10.1093/jn/135.2.348. [DOI] [PubMed] [Google Scholar]
- 7.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 8.Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, Packer L, Traber MG, Farese RV., Jr Increased atherosclerosis in hyperlipidemic mice deficient in α tocopherol transfer protein and Vitamin E. PNAS. 2000;97:13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Hung Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2:RESEARCH0032. doi: 10.1186/gb-2001-2-8-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vleet JF, Ferrans VJ, Ruth GR. Ultrastructural alterations in nutritional cardiomyopathy of selenium-vitamin E deficient swine. I Fiber lessons. Lab Invest. 1977;37:188–200. [PubMed] [Google Scholar]
- 11.Lin CT, Chen LH. Ultrastructural and lysosomal enzyme studies of skeletal muscle and myocardium in rats with long-term vitamin E deficiency. Pathology. 1982;14:375–382. doi: 10.3109/00313028209092115. [DOI] [PubMed] [Google Scholar]
- 12.Doni MG, Falanga A, Delaini F, Vicenzi E, Tomasiak M, Donati MB. The effect of vitamin E or selenium on the oxidant-antioxidant balance in rats. Br J Exp Pathol. 1984;65:75–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger SK, Cross CE, Williams DE. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic Biol Med. 2005;38:773–785. doi: 10.1016/j.freeradbiomed.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Mickle DAG, Weisel RD, Burton GW, Ingold KU. Effect of orally administered alpha-tocopheryl acetate on human myocardial alpha-tocopherol levels. Cardiovasc Drugs Ther. 1991;5:309–312. doi: 10.1007/BF00054753. [DOI] [PubMed] [Google Scholar]
- 15.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue α-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 16.Arita M, Sato Y, Miyata A, Tanabe T, Takahashi E, Kayden HJ, Arai H, Inoue E. Human α-tocopherol transfer protein: cDNA cloning, expression and chromosomal localization. J Biochem. 1995;306:437–443. doi: 10.1042/bj3060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gohil K, Godzdanker R, O’Roark E, Schock BC, Kaini RR, Packer L, Cross CE, Traber MG. Alpha-tocopherol transfer protein deficiency in mice causes multi-organ deregulation of gene networks and behavioral deficits with age. Ann NY Acad Sci. 2004;1031:109–126. doi: 10.1196/annals.1331.012. [DOI] [PubMed] [Google Scholar]
- 18.Gohil K, Oommen S, Vasu VT, Aung HH, Cross CE. Tocopherol transfer protein deficiency modifies nuclear receptor transcriptional networks in lungs: modulation by cigarette smoke in vivo. Mol Aspects Med. 2007 doi: 10.1016/j.mam.2007.02.004. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney CW, Azzi A. Vitamin E inhibits protein kinase C activity. Biochem Biophys Res Commun. 1988;154:694–697. doi: 10.1016/0006-291x(88)90195-7. [DOI] [PubMed] [Google Scholar]
- 20.Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C J Biol Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- 21.Freedman JE, Farhat JH, Loscalzo J, Keaney JF., Jr Alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation. 1996;94:2434–2440. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 22.Chatelain E, Boscoboinik DO, Bartoli GM, Kagan VE, Gey FK, Packer L, Azzi A. Inhibition of smooth muscle cell proliferation and protein kinase C activity by tocopherols and tocotrienols. Biochim Biophys Acta. 1993;1176:83–89. doi: 10.1016/0167-4889(93)90181-n. [DOI] [PubMed] [Google Scholar]
- 23.Tasinato A, Boscoboinik D, Bartoli GM, Maroni P, Azzi A. d-alpha-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc Natl Acad Sci USA. 1995;92:12190–12194. doi: 10.1073/pnas.92.26.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozer NK, Palozza P, Boscoboinik D, Azzi A. d-α-tocopherol inhibits low density lipoprotein induced proliferation and protein kinase C activity in vascular smooth muscle cells. FEBS Lett. 1993;322:307–310. doi: 10.1016/0014-5793(93)81592-n. [DOI] [PubMed] [Google Scholar]
- 25.Suarna C, Dean RT, May J, Stocker R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of α-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 26.Hazell LJ, Stocker R. α-Tocopherol does not inhibit hypochlorite-induced oxidation of apolipoprotein B-100 of low-density lipoprotein. FEBS Lett. 1997;414:541–544. doi: 10.1016/s0014-5793(97)01066-1. [DOI] [PubMed] [Google Scholar]
- 27.Terentis AC, Thomas SR, Burr JA, Liebler DC, Stocker R. Vitamin E oxidation in human atherosclerotic lesions. Circ Res. 2002;90:333–339. doi: 10.1161/hh0302.104454. [DOI] [PubMed] [Google Scholar]
- 28.Meydani SN, Tengerdy RP. In: Vitamin E: Biochemical and Clinical Applications. Packer L, Fuchs J, editors. Marcel Dekker; New York: 1991. p. 549. [Google Scholar]
- 29.Beharka A, Redican S, Leka L, Meydani SN. Vitamin E status and immune function. Methods Enzymol. 1997;282:247–263. doi: 10.1016/s0076-6879(97)82112-x. [DOI] [PubMed] [Google Scholar]
- 30.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SN, Wu WD, Ha WK, Beharka A, Smith DE, Bender BS, Meydani SN. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marco MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J Immunol. 2007;178:1443–1449. doi: 10.4049/jimmunol.178.3.1443. [DOI] [PubMed] [Google Scholar]
- 33.Beck MA, Kolbeck PC, Rohr LH, Shi Q, Morris VC, Levander OA. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J Nutr. 1994;124:345–358. doi: 10.1093/jn/124.3.345. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Ma M, Ippolito GC, Schroeder HW, Jr, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci USA. 2001;98:14577–14582. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- 36.Tomee JF, Koeter GH, Hiemstra PS, Kauffman HF. Secretory leukoprotease inhibitor: a native antimicrobial protein presenting a new therapeutic option? Thorax. 1998;53:114–116. doi: 10.1136/thx.53.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canaan A, Yu X, Booth CJ, Lian J, Lazar I, Gamfi SL, Castille K, Kohya N, Nakayama Y, Liu YC, Evnon E, Flavell R, Weissman SM. FAT10/diubiquitin-like protein-deficient mice exhibit minimal phenotypic differences. Mol Cell Biol. 2006;26:5180–5189. doi: 10.1128/MCB.00966-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal. 1998;10:699–711. doi: 10.1016/s0898-6568(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 39.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 40.Brade T, Manner J, Kuhl M. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc Res. 2006;72:198–209. doi: 10.1016/j.cardiores.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Stam H, Schoonderwoerd K, Hulsmann WC. Synthesis, storage and degradation of myocardial triglycerides. Basic Res Cardiol. 1987;82:19S–28S. doi: 10.1007/978-3-662-08390-1_3. [DOI] [PubMed] [Google Scholar]
- 42.Ntambi JM, Bene H. Polyunsaturated fatty acid regulation of gene expression. J Mol Neurosci. 2001;16:273–278. doi: 10.1385/JMN:16:2-3:273. [DOI] [PubMed] [Google Scholar]
- 43.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 44.Garlid KD, Jaburek M, Jezek P, Varecha M. How do uncoupling proteins uncouple? Biochimica Biophysica Acta. 2000;1459:383–389. doi: 10.1016/s0005-2728(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 45.De Meyer SF, Vanhoorelbeke K, Ulrichts H, Staelens H, Feys HB, Salles I, Fontayne A, Deckmyn H. Development of monoclonal antibodies that inhibit platelet adhesion or aggregation as potential anti-thrombotic drugs. Cardiovasc Hematol Disord Drug Targets. 2006;6:191–207. doi: 10.2174/187152906778249536. [DOI] [PubMed] [Google Scholar]
- 46.Abedin M, Omland T, Ueland T, Khera A, Aukrust P, Murphy SA, Jain T, Gruntmanis U, McGuire DK, de Lemos JA. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study) Am J Cardiol. 2007;99:513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 47.Sasson S, Eckel J. Disparate effects of 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid in vascular endothelial and smooth muscle cells and in cardiomyocytes. Arch Physiol Biochem. 2006;112:119–129. doi: 10.1080/13813450600712035. [DOI] [PubMed] [Google Scholar]
- 48.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 49.Baltus T, von Hundelshausen P, Mause SF, Buhre W, Rossaint R, Weber C. Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. J Leukoc Biol. 2005;78:435–441. doi: 10.1189/jlb.0305141. [DOI] [PubMed] [Google Scholar]
- 50.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem. 2005;280:21997–22005. doi: 10.1074/jbc.M413676200. [DOI] [PubMed] [Google Scholar]
- 51.Schock BC, van der Vliet A, Corbacho AM, Leonard SW, Finkelstein E, Valacchi G, Obermueller-Jevic U, Cross CE, Traber MG. Enhanced inflammatory responses in alpha-tocopherol transfer protein null mice. Arch Biochem Biophys. 2004;423:162–169. doi: 10.1016/j.abb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Keeley FW, Sitarz EE. Identification and quantitation of alpha 2-HS-glycoprotein in the mineralized matrix of calcified plaques of atherosclerotic human aorta. Atherosclerosis. 1985;55:63–69. doi: 10.1016/0021-9150(85)90166-2. [DOI] [PubMed] [Google Scholar]
- 53.Eckstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P450 (P450 IIE1) Biochemical Pharmacology. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 54.Marzouki N, Benomar A, Yahyaoui M, Birouk N, Elouazzani M, Chkili T, Benlemlih M. Vitamin E deficiency ataxia with (744 del A) mutation on α-TTP gene: genetic and clinical peculiarities in Moroccan patients. Eur J Med Genet. 2005;48:21–28. doi: 10.1016/j.ejmg.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Ricciarelli R, Zing J-M, Azzi A. Vitamin E: protective role of a Janus molecule. FASEB J. 2001;15:2314–2325. doi: 10.1096/fj.01-0258rev. [DOI] [PubMed] [Google Scholar]
- 56.Azzi A, Gysin R, Kempna P, Munteanu A, Villacorta L, Visarius T, Zing JM. Regulation of gene expression by α-tocopherol. Biol Chem. 2004;385:585–591. doi: 10.1515/BC.2004.072. [DOI] [PubMed] [Google Scholar]