Abstract
Lymphatic vessels are essential for lipid absorption and transport. Despite increasing numbers of observations linking lymphatic vessels and lipids, little research has been devoted to address how dysregulation of lipid balance in the blood, ie, dyslipidemia, may affect the functional biology of lymphatic vessels. Here, we show that hypercholesterolemia occurring in apolipoprotein E-deficient (apoE−/−) mice is associated with tissue swelling, lymphatic leakiness, and decreased lymphatic transport of fluid and dendritic cells from tissue. Lymphatic dysfunction results in part from profound structural abnormalities in the lymphatic vasculature: namely, initial lymphatic vessels were greatly enlarged, and collecting vessels developed notably decreased smooth muscle cell coverage and changes in the distribution of lymphatic vessel endothelial hyaluronic acid receptor-1 (LYVE-1). Our results provide evidence that hypercholesterolemia in adult apoE−/− mice is associated with a degeneration of lymphatic vessels that leads to decreased lymphatic drainage and provides an explanation for why dendritic cell migration and, thus, immune priming, are compromised in hypercholesterolemic mice.
Lymphatic vessels are essential for maintaining tissue fluid balance, facilitating immune cell trafficking from the periphery to lymph nodes, and absorbing lipoprotein from the gut and from tissue adipocytes.1,2 Although these three functions are likely to be interconnected, their interdependence has not been well established. When lymphatic vessels are malformed or dysfunctional, swelling occurs, which, when chronic, can lead to dermal lipid accumulation3,4 and impaired immune responses.5,6 In mice heterozygous for the homeobox gene Prox1, which is essential for the development of lymphatic vasculature, abnormal lymph leakage correlated with disrupted integrity of the lymphatic vasculature, particularly in the valves. This leakage leads to increased lipid storage in adipocytes and adipogenesis.7 Conversely, patients with lipedema, a vascular disease characterized by edema and increased subcutaneous adipose tissue in the legs, present with microlymphatic aneurysms8 and functional lymphatic alterations similar to those found in patients with lymphedema.9
Despite these clinical data linking lipid homeostasis to lymphatic vessels, little basic research has been devoted to address how dysregulation of the lipid balance in the blood, ie, dyslipidemia, may affect the functional biology of lymphatic vessels. Dyslipidemia that includes elevated levels of total cholesterol in the form of low-density lipoprotein (hypercholesterolemia) with correspondingly decreased levels of high-density lipoprotein is a major risk factor for atherosclerosis and a common clinical feature of autoimmune diseases10 and certain cancers.11 Dyslipidemia in mice is associated with significant systemic manifestations that lead to marked inflammatory changes in many tissues, especially skin.12,13,14,15 Cholesterol accumulation in the skin also occurs in humans with severe familial disease,16 and it is well established that dyslipidemia not only promotes atherosclerotic disease but causes a systemic dysfunction of the blood vasculature in man and animal models.17 Here, we show, using mice lacking apolipoprotein E (apoE−/−) as a model of dyslipidemia, that this systemic dysfunction extends to the lymphatic vasculature as well, because lymphatic vessel integrity and transport function declined as hypercholesterolemia advanced.
Materials and Methods
Animals
Male wild-type and apoE−/− mice fully backcrossed on the C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME). Both wild-type and apoE−/− mice were maintained on a chow diet (18% protein and >5% fat, Harlan Teklad, Madison, WI) when stated or otherwise switched at 6 weeks of age to a high-fat diet (21% milk fat and 0.15% cholesterol; Harlan Teklad) for 10 to 13 weeks or for an additional 24 to 29 weeks, corresponding to 16 to 19 and 30 to 35 weeks of age, respectively. All studies were approved by the institutional animal care and use committees at Mt. Sinai School of Medicine, National University of Singapore, and the Veterinary Authorities of the Canton Vaud. Total plasma cholesterol was measured with a commercial kit (BioVision, Mountain View, CA).
Immunohistochemistry
Cryosections (6 to 8 μm) from skin were prepared as described previously.18 For histological analysis, sections were stained with hematoxylin and eosin or oil red O. For immunohistochemistry, primary antibodies used included rabbit anti-lymphatic vessel endothelial hyaluronic acid receptor-1 (LYVE-1) polyclonal antibody (Upstate Biotechnology, Charlottesville, VA), hamster anti-podoplanin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and biotinylated anti-CD45 antibodies. Cy3- or Alexa 647-conjugated anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA) and Alexa 488-conjugated anti-hamster (Invitrogen, Carlsbad, CA) antibodies and Cy3-conjugated streptavidin were used for detection. Sections were counterstained with 4,6-diamidino-2-phenylindole for cell nuclei visualization and mounted for analysis.
Whole-mount immunohistochemical analysis of ear skin and intestine was performed as described previously.7 Mice were perfused with 4% paraformaldehyde, and tissue was dissected and further fixed in 4% paraformaldehyde overnight at 4°C. Then tissues were incubated in blocking solution 0.5% bovine serum albumin and 0.3% Triton X-100 in PBS overnight at 4°C and finally incubated with anti-LYVE-1 and anti-podoplanin antibodies followed by Cy3- or Alexa 647-conjugated anti-rabbit and Alexa 488-conjugated anti-hamster antibodies. In some experiments, whole mounts were also stained with Cy3-conjugated anti-smooth muscle actin (Sigma-Aldrich, St. Louis, MO) or with anti-rat CD31 (PECAM-1; BD Biosciences, San Jose, CA) revealed by Cy3-conjugated anti-rat antibody (Jackson ImmunoResearch) to analyze smooth muscle cell coverage and valves on collecting vessels, respectively.19 Specimens were viewed with a fluorescence (Axio imager.Z1, Axiocam HRM camera; Carl Zeiss Micro Imaging, Inc., Jena, Germany) or confocal microscope (Leica TCS SP5; Leica Microsystems, Inc., Deerfield, IL) using LAS AF confocal software (version 1.8.2; Leica Microsystems, Inc.).
To determine the diameter of LYVE-1+ vessels, images of representative whole mounts stained for LYVE-1 were acquired on a Zeiss fluorescence microscope with a Zeiss MRm camera, and computer-assisted morphometric analysis of initial lymphatic vessels was performed using Metamorph 6.3 software (Molecular Devices, Toronto, ON, Canada). Linear vessel segments were outlined using a freehand drawing tool and Wacom monitor; vessel junctions were avoided. The average vessel diameter was then calculated from each highlighted vessel by the software; 10 images were used from each mouse (n = 5 to 7 for each condition).
Lymphatic Functional Analysis
Evans blue (1% w/v) dye was injected at the inner surface of the rim of the ear from anesthetized mice using a 10-μl Hamilton syringe, a standard method to macroscopically visualize cutaneous lymphatic vessels and lymphatic drainage.20 Mouse ears were photographed within 1 minute after dye injection. The functional uptake of the initial lymphatic vessels in the tail was determined by adapting the technique of fluorescence microlymphangiography.4,21,22 In brief, a 30-gauge needle catheter containing 0.9% NaCl with 1% fluorescein isothiocyanate-conjugated dextran (70 kDa) was placed intradermally into the tip of the tail. The catheter was attached to a low-pressure reservoir that permitted changes of infusion pressures of 40, 45, 50, and 55 cm H2O. The low infusion pressure allowed uptake into the lymphatic capillaries without deformation of the vessels or changes in their function due to high pressure. The fluorescent dextran, once in the tail, either travels through the interstitial space (linearly with pressure change) or is taken up and transported by the lymphatic capillaries. The infusion flow rate was continually monitored; then pressures were changed and flow rates were monitored for 30 minutes per pressure setting. By also monitoring the diffusive front of fluorescence at each infusion pressure, using a fluorescence microscope, the hydraulic conductivity of the matrix and than that of the lymphatic conductance were calculated.21,22
Dendritic Cell Migration Assay
For adoptive transfer of dendritic cells (DCs), spleens from CD45.1+ wild-type or CD45.2+ apoE−/− congenic mice, respectively, were isolated and digested with collagenase D as described previously for lymph nodes.23 Then CD11c+ DCs were isolated using a magnetic microbead-based, positive-selection kit (Miltenyi Biotech, Gladbach, Germany). Wild-type and apoE−/− DCs were mixed in a nearly equal ratio, and this “preinjection” ratio (CD45.1+ DC frequency/CD45.2+ DC frequency) was recorded by fluorescence-activated cell sorting. Mixed DC suspensions (8 × 105 cells) were injected into each side of the scapular skin of wild-type CD45.1 × CD45.2 F1 recipient mice, using at least four recipient mice per experiment. After 36 hours, cell suspensions from brachial lymph nodes were stained for CD11c and CD45.1 or CD45.2. To obtain a “postinjection” ratio of DCs, total CD11c+ cells were gated, CD45.1+CD45.2+ double-positive host cells were ignored, and the frequency of CD45.1+ and CD45.2+ single-positive DCs was determined. These frequencies were used to tally the postinjection ratio of DCs (wild-type CD45.1+ cells/apoE−/− CD45.2+ cells). In other experiments (Figure 1C), CD45.1+ wild-type DCs were injected into CD45.2+ wild-type or CD45.2+ apoE−/− recipient mice, and the total number of transferred DCs that migrated into lymph nodes was determined by multiplying the percentage of CD11c+ congenic DCs observed in the lymph node suspension by the number of total lymph node cells.
Figure 1.
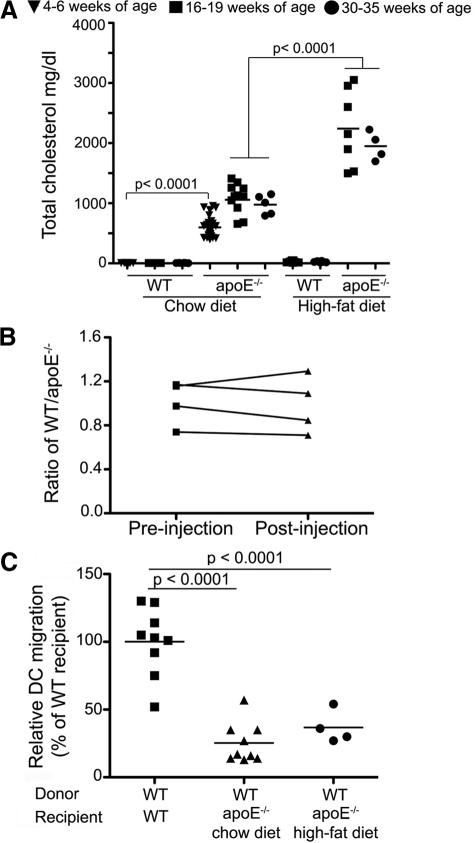
Hypercholesterolemia and impaired dendritic cell migration in apoE−/− mice. A: Plasma cholesterol levels were analyzed from wild-type (WT) and apoE−/− mice at 4 to 6, 16 to 19, and 30 to 35 weeks of age. Some were fed a high-fat diet at 6 week of age. B: Splenic DCs derived from congenic CD45.1+ wild-type and CD45.2+ apoE−/− mice, respectively, were mixed in a near equal ratio (Pre-injection; CD45.1+ DCs/CD45.2+ DCs) and adoptively transferred to the scapular skin of wild-type CD45.1+CD45.2+ double-positive F1 recipient mice. The ratio of CD45.1 single-positive DCs to CD45.2 single-positive DCs was determined 36 hours later in the lymph node (Post-injection). Four experiments are shown, with no statistical difference between Pre-injection versus Post-injection ratios. C: A constant number of CD45.1+ wild-type splenic DCs was transferred into CD45.2+ wild-type or CD45.2+ apoE−/− recipient mice, and the total number of migrated DCs recovered from draining lymph nodes was determined 36 hours after cell transfer. Four to eight animals were independently analyzed in each group.
Statistical Analysis
Statistical analysis was performed with a two-tailed nonparametric Mann-Whitney U test for single comparisons or analysis of variance with a post hoc test using Bonferroni’s method for multiple comparisons. P < 0.05 was considered significant.
Results
Sign of Lymphatic Alterations in apoE−/− Mice
As a constituent of triglyceride-rich plasma lipoproteins, apoE serves as an important ligand for lipoprotein recognition and clearance by lipoprotein receptors. Consequently, homozygous deletion of the apoE gene in mice results in a pronounced increase in the plasma levels of cholesterol.24,25,26 As reported previously,27 apoE-deficient mice (apoE−/−) fed a standard chow diet typically developed spontaneous hypercholesterolemia, and their plasma cholesterol reached 600 to 900 mg/dl at and from 6 weeks of age (Figure 1A). In contrast, age-, sex-, and diet-matched wild-type mice had cholesterol levels of <100 mg/dl (Figure 1A). Consistent with other reports,24,28 a high-cholesterol, high-fat diet significantly accelerated and exacerbated the accumulation of blood cholesterol in apoE−/− mice, with levels rising to 2 to 3 times that of apoE−/− mice fed a chow diet (Figure 1A). Previously, we demonstrated that apoE−/− mice from 16 weeks of age exhibit leukocyte accumulation in skin and compromised migration of endogenous skin DCs into draining lymph nodes.15 However, in this latter report, we did not determine whether the defect developed autonomously within DCs or whether and how environmental conditions (eg, lymphatic drainage) contributed.
To address this point, we adoptively transferred splenic CD11c+ cells, highly enriched in DCs (supplemental Figure S1, http://ajp.amjpathol.org), derived from CD45.2 wild-type and CD45.1 hypercholesterolemic apoE−/− mice into skin of wild-type CD45.1/2 F1 recipient mice, or, conversely, CD11c+ splenic DCs derived from CD45.2 wild-type mice were transferred into wild-type or apoE−/− congenic CD45.1 recipient mice fed either a chow or high-fat diet. Migration of the transferred cells to the skin-draining lymph node was analyzed 36 hours later. When injected into wild-type mice with a low-cholesterol environment, the migration of apoE−/− DCs was similar to that of wild-type DCs, as assessed by coinjecting wild-type and apoE−/− CD11c+ DCs in an equal ratio and assaying their ratio within draining lymph nodes (Figure 1B). Thus, neither the lack within DCs of the apoE gene nor their preconditioning in a hypercholesterolemic environment directly affected DC migration from skin into lymphatic vessels. In contrast, wild-type DCs injected into apoE−/− mice showed substantially decreased migration compared with their migration in wild-type recipients, regardless of their diet (Figure 1C). These findings reveal that the impaired DC migration to lymph nodes that occurs during hypercholesterolemia results from modifications within the environment of the migrating DCs, rather than effects on DCs themselves. Because lymphatic vessels are essential for trafficking of DCs,29 we hypothesized that these environmental modifications might include alterations in the lymphatic vasculature.
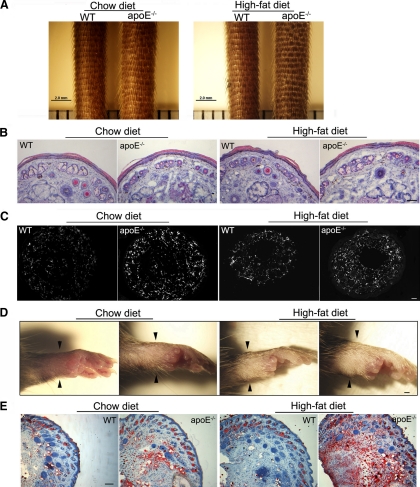
Consistent with this hypothesis, we found that apoE−/− mice exhibited tissue swelling, which can result from the impaired lymphatic drainage of interstitial fluid. Tail diameters from apoE−/− mice were larger than those of age-, sex-, and diet-matched wild-type controls (Table 1). At 16 weeks of age, swelling of the tail was visible macroscopically in apoE−/− mice fed a chow or high-fat diet (Figure 2A). Hematoxylin and eosin stains of tail skin sections from apoE−/− mice and matched controls revealed the appearance of numerous “open” areas that probably result from the accumulation of extracellular fluid20,30 in the dermis of 20-week-old apoE−/− mice fed either a chow or high-fat diet compared with wild-type mice (Figure 2B). In addition, this dermal edema in apoE−/− mice was associated with a notably increased number of CD45+ leukocytes (Figure 2C). Swelling in the footpad of apoE−/− mice fed a chow or high-fat diet was also evident by 20 weeks of age (Figure 2D), and oil red O staining revealed that this footpad swelling was, in part, associated with lipid deposition (Figure 2E). This last finding is consistent with a previous study reporting cholesterol accumulation in skin from atherosclerotic mice.14
Table 1.
Tail diameters measured 4 cm from the tip, in wild-type and apoE−/− mice at 6 and 16 weeks of age
| Strain | Tail diameter (mm)
|
||
|---|---|---|---|
| 6 weeks of age | 16 weeks of age fed a chow diet | 16 weeks of age fed a high-fat diet | |
| Wild-type | 2.32 ± 0.06 | 2.40 ± 0.04 | 2.36 ± 0.08 |
| apoE−/− | 2.23 ± 0.05 | 2.55 ± 0.11* | 2.56 ± 0.08* |
Some mice were fed a high-fat diet from 6 weeks of age. Values are shown as mean ± SD.
Significant differences from wild-type controls: P < 0.0001 (n = 3 to 11).
Figure 2.
Tissue swelling in hypercholesterolemic apoE−/− mice. Tail swelling was visible 4 cm from the tips in 16-week-old apoE−/− mice fed either a chow or high-fat diet (scale bar = 2 mm) (A) and was associated with peripheral edema (B) and CD45+ leukocyte infiltration (C). Scale bars: 2 mm (A); 50 μm (C). Footpad swelling (arrowheads) was also observed in apoE−/− mice fed a chow or high-fat diet by 20 weeks of age (D) and resulted, at least in part, from lipid accumulation as revealed by oil red O staining of footpad skin sections (E). Scale bars = 50 μm. WT, wild-type.
Decreased Lymphatic Function in apoE−/− Mice
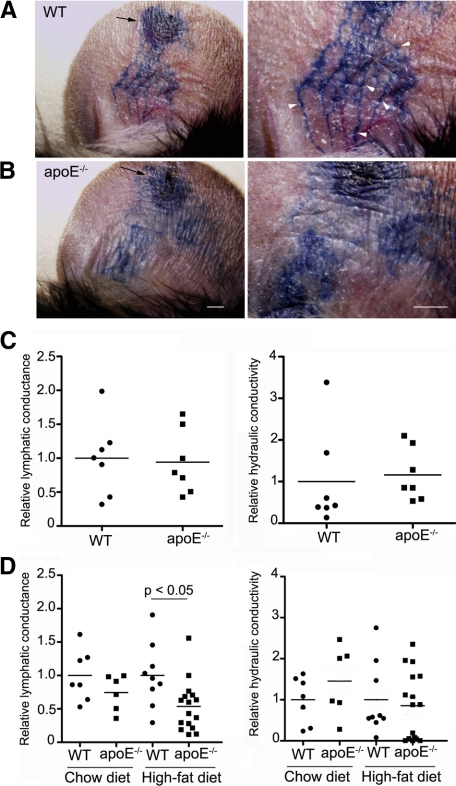
We next specifically assessed the lymphatic transport of macromolecules in apoE−/− mice. As a first approach, we examined the transport of Evans blue dye, which binds interstitial proteins such as albumin and is taken up into the lymphatic vessels, after intradermal injection into the ear. In 16-week-old wild-type mice fed a high-fat diet, we could visualize fine, distinct lymphatic capillaries as the dye was taken up into the vessels and transported toward the base of the ear (Figure 3A). In contrast, when injected into the ear of 16-week-old apoE−/− mice fed a high-fat diet, dye was poorly taken up into lymphatic capillaries and appeared to leak out into the surrounding interstitial space such that lymphatic capillaries could not be clearly delineated (Figure 3B). Because lymph leakage has typically been associated with dysfunctional valves,7,31 this observation suggested that lymphatic vessels themselves were dysfunctional in the hypercholesterolemic mice.
Figure 3.
Impaired lymphatic vascular function in hypercholesterolemic mice. A, B: Evans blue dye was injected intradermally into the ear rim of 16- to 18-week-old wild-type (WT) and apoE−/− mice (arrows indicate the site of injection). After 1 minute, markedly dilated lymphatic vessels were visualized in apoE−/− mice ears, and Evans blue dye had extravasated from these dilated lymphatic vessels (B) compared with matched wild-type control mice, where fine lymphatic vessels were observed (arrowheads, A). C, D: Lymphatic uptake (conductance) and ease of fluid movement through the interstitial space (hydraulic conductivity) were analyzed by skin tail microlymphangiography in wild-type and apoE−/− mice at 4 to 5 weeks of age (C) or in older wild-type and apoE−/− mice maintained on a chow diet or fed a high-fat diet from 6 weeks of age (D). For each group, n ≥ 6.
We next quantified the functional uptake of the initial lymphatic vessels in tail skin, which exhibit a strikingly regular hexagonal network that is easily visualized by fluorescence microlymphangiography after fluorescent dextran, used as a lymphatic tracer, is introduced into the tip of the tail at constant pressure.22 By comparing how flow into the dermis changes with infusion pressure, and simultaneously how tracer movement in the dermis and into the lymphatic capillaries changes with infusion pressure, we can derive an in situ measurement of hydraulic conductivity (representing how easily fluid can move through the extracellular matrix) and lymphatic conductance (representing how readily lymphatics drain interstitial fluid for a given tissue fluid pressure). This method has demonstrated utility for the detection of impaired lymphatic function in established models of lymphedema.4,22
In young, 4- to 5-week-old apoE−/− mice (Figure 3C), we observed no difference in lymphatic conductance or hydraulic conductivity relative to that in age-matched wild-type controls (Figure 3C). In contrast, the capacity of lymphatics to take up the injected fluorescent tracer in apoE−/− mice was reduced by approximately 50% in apoE−/− mice fed a high-fat diet and examined at 16–18 weeks of age (Figure 3D, left panel). This defect was not associated with any changes in tissue structure as seen by the hydraulic conductivity (Figure 3D, right panel). However, apoE−/− mice fed a chow diet did not show a significant defect in lymphatic conductance (Figure 3D, left panel). Because DC migration is dramatically and similarly compromised in apoE−/− mice fed either a chow or a high-fat diet, it was possible that other defects in lymphatic vessels contributed to the impaired DC migration in apoE−/− mice, in addition to decreased lymphatic conductance within the initial lymphatic capillary plexus measured here. Thus, we further investigated the morphology and the structure of initial and collecting lymphatic vessels.
Enlarged Initial Lymphatic Vessels in apoE−/− Mice
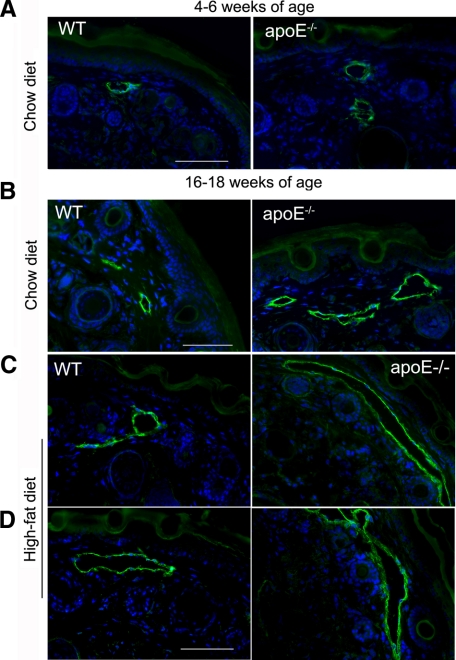
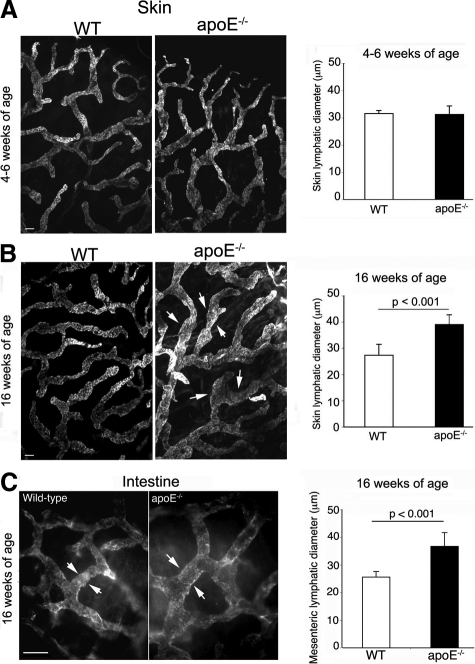
Because lymphedematous tissue often displays hyperplastic lymphatic vessels,4 we investigated the morphology of initial lymphatic vessels that lie in the subepidermal region using immunofluorescence with LYVE-1 and podoplanin (supplemental Figure S2, see http://ajp.amjpathol.org). At 6 weeks of age, initial lymphatic vessels in tail skin from wild-type and apoE−/− mice appeared similarly normal, ie, in a partially collapsed state (Figure 4A), consistent with the expected slow passage of fluid through resting tissue. In contrast, in 16-week-old apoE−/− mice, the majority of initial lymphatic vessels were notably dilated with open lumens compared with those in age-matched wild-type mice (Figure 4, B–D). Consistent with the exacerbating effects of the high-fat diet on blood cholesterol levels, we found that this high-fat diet furthered the enlargement of initial lymphatic vessels in apoE−/− mice compared with that in apoE−/− mice fed a chow diet (Figure 4, C and D). In wild-type mice fed a high-fat diet, the size of the initial lymphatic vessels was generally similar to that in young or 16-week-old wild-type mice fed a chow diet with occasional lymphatic vessels appearing slightly dilated; overall the dilation appeared minimal compared with that seen in apoE−/− mice. Similarly, enlargement of initial lymphatic vessels was observed in ear skin of apoE−/− mice fed a high-fat diet (supplemental Figure S3, http://ajp.amjpathol.org). These findings are consistent with the lymphatic hyperplasia seen in skin lymphedema.4
Figure 4.
Morphological changes of initial lymphatics in apoE−/− mice. (A) At 4 to 6 weeks of age, the morphology of initial podoplanin-positive lymphatic vessels in tail skin from apoE−/− mice was similar to that in wild-type (WT) mice. In contrast, at 16 to 18 weeks of age, initial lymphatics in apoE−/− mice fed a chow diet were dilated with wide lumens (B). This lymphatic enlargement was further exacerbated in apoE−/− mice fed a high-fat, rich-cholesterol diet (C, D). For each group, n = 2 to 6. Scale bars = 50 μm.
Whole-mount immunofluorescence for LYVE-1 of ear skin from wild-type and apoE−/− mice further illustrated the dilation or hyperplasia of initial lymphatic vessels in 16-week-old apoE−/− mice fed a high-fat diet (Figure 5, A and B). Indeed, the lymphatic vessel diameters were significantly increased compared with those in wild-type controls (Figure 5B). In contrast, the morphology of initial lymphatic vessels from 4- to 6-week-old apoE−/− mice with minimal hypercholesterolemia was similar to that in young wild-type mice (Figure 5A). These lymphatic changes in hypercholesterolemic mice were not restricted to cutaneous lymphatic vessels, as vessel hyperplasia was also observed in the lymphatic vessels of the intestine (Figure 5C).
Figure 5.
Systemic lymphatic hyperplasia in hypercholesterolemic mice. A–C: Immunostaining for LYVE-1 was examined in whole mounts of ear skin from wild-type (WT) and apoE−/− mice at 4 to 6 weeks of age (A) or in wild-type and 16 week-old apoE−/− mice maintained on a high-fat diet from 6 weeks of age (B, C), and the diameter of LYVE-1+ vessels was quantified (A–C, arrowheads) and expressed as mean ± SD Hyperplasia of lymphatics was not restricted to skin but was apparently systemic as lymphatic vessels in intestine of 16-week-old apoE−/− mice fed a high-fat diet were significantly dilated (C, arrowheads). For each group, n = 5 to 6. Scale bars = 50 μm.
Loss of Collecting Vessel Identity in apoE−/− Mice
As revealed by examination of Evans blue transport, lymphatic vessels in apoE−/− mice were leaky, because they were only faintly delineated and blue dye was seen in the surrounding interstitium (Figure 3). These observations prompted us to analyze the morphology and structure of collecting vessels in these mice.
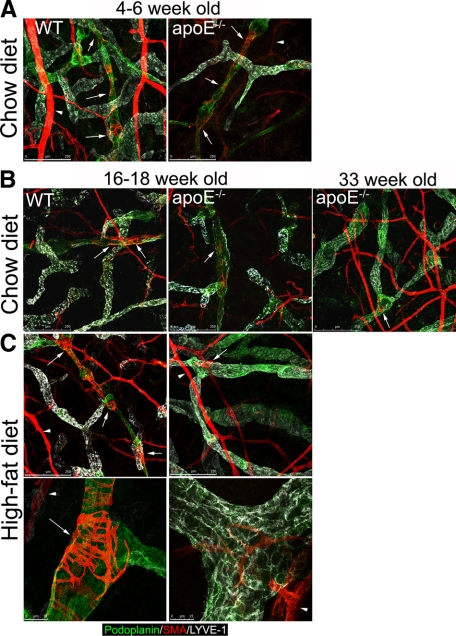
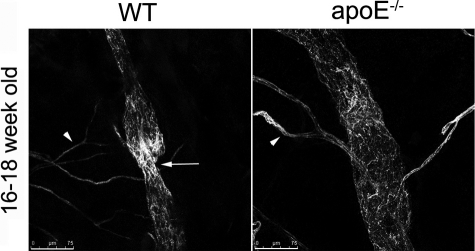
In 4- to 6-week-old apoE−/− and wild-type mice, collecting vessels appeared normal in whole-mount immunostaining of ear skin: they expressed podoplanin but down-regulated LYVE-1, which remains high in the initial lymphatic vessels, and they exhibited normal smooth muscle cell coverage and characteristic intraluminal valves (Figure 6A and data not shown). In contrast, in older hypercholesterolemic apoE−/− mice fed either a chow or high-fat diet, these distinctions between initial and collecting vessels were not evident (Figure 6, B and C). The collecting vessels were strongly positive for LYVE-1, and little or no smooth muscle cell coverage was observed on these hyperplastic lymphatic vessels as revealed by confocal microscopy (Figure 6, B and C). In comparison with apoE−/− mice fed a high-fat diet, the dysregulation of LYVE-1 expression on collecting vessels was only observed by 33 weeks of age in apoE−/− mice fed a chow diet although these mice already exhibited the loss in smooth muscle cell coverage by 16 weeks of age. The defect in smooth muscle cell coverage appeared to be restricted to the lymphatic vasculature because blood vessels in the same hypercholesterolemic mice were strongly positive for smooth muscle actin (Figure 6, B and C). Furthermore, although lymphatic vessels in older, hypercholesterolemic apoE−/− mice stained positive for CD31, they did not exhibit the normal valve structure as observed in age- and diet-matched wild-type mice (Figure 7) and described previously.19 These abnormal valves may explain the reflux of Evans blue from lymphatic vessels observed in ear skin of hypercholesterolemic mice because valves normally prevent back flow (Figure 3). Altogether, these data suggest that chronic hypercholesterolemia in apoE−/− mice is associated with degeneration of collecting vessels by loss of smooth muscle coverage, which in turn contributes to poor lymphatic transport and impaired DC migration.
Figure 6.
Hypercholesterolemia in apoE−/− mice is associated with abnormal collecting vessels. Immunostaining for LYVE-1, podoplanin, and smooth muscle actin (SMA) in ear skin whole mounts was performed to identify collecting vessels in wild-type (WT) and apoE−/− mice at 4 to 6 (A) or 16 to 33 weeks of age maintained on a chow or high-fat diet (B, C). As observed in age-matched wild-type mice, LYVE-1 expression remained high in initial lymphatics of 4- to 6-week-old apoE−/− mice (A), and LYVE-1 was down-regulated or abrogated on podoplanin-positive collecting vessels, which were covered with smooth muscle cells (arrows). As expected, the coverage with smooth muscle actin-expressing cells of lymphatic vessel was sparser (arrows) than in the blood vessels (arrowheads). In contrast, podoplanin-positive lymphatic vessels in 16- to 33-week-old apoE−/− mice fed a chow or high-fat diet failed to down-regulate LYVE-1 marker (B) and had a reduced smooth muscle cell coverage (B, C). For each group, n = 2 to 4. Scale bars, 250 μm (A, B, C, top panel); 25 μm (C, bottom panel).
Figure 7.
Loss of valve in collecting vessels from apoE−/− mice. Immunoreactivity for CD31 revealed intraluminal valves (arrow) in collecting vessels from wild-type (WT) mice that were not apparent in hypercholesterolemic apoE−/− mice maintained on a high-fat diet. As expected, blood vessels stained for CD31 (arrowheads). For each group, n = 2 to 4. Scale bars = 75 μm.
Discussion
Here, we found that the morphology and function of lymphatic vessels in the skin of apoE−/− mice were normal at 6 weeks of age, but beyond 16 weeks, when hypercholesterolemia was marked, lymphatic vessels became hyperplastic and collecting vessels failed to down-regulate the initial lymphatic capillary marker LYVE-1, lost coverage with smooth muscle cells, and showed abnormal valves. Thus, lymphatic vessels develop and mature normally in apoE−/− mice, but they degenerate and lose their function, apparently in response to hypercholesterolemia, although we cannot eliminate the possibility that the primary defect is a lack of apoE that becomes manifest late in life. However, because we show here that impaired DC migration correlates with loss of lymphatic vessel integrity and impaired DC migration is known to develop in both hypercholesterolemic apoE−/− and LDLR−/− mice,15 it is likely that hypercholesterolemia itself rather than the absence of apoE accounts for the changes in lymphatic structure we describe. It is known that total cholesterol and apolipoprotein levels in human lymph and interstitial fluid are inversely correlated with lymph flow rates.32 However, this study is, to our knowledge, the first report of structural and functional abnormalities of lymphatic vasculature associated with hypercholesterolemia and furthermore provides evidence that remodeling of the lymphatic vasculature is possible in adult mice under pathological conditions.
The data raise the possibility of a two-way relationship between lymphatic function and cholesterol homeostasis, with the potential to set up a vicious cycle: elevated low-density lipoprotein or associated metabolites may decrease lymphatic function in tissues, and this loss of function in turn may render the tissue susceptible to still further and more profound accumulation of cholesterol. Impaired lymphatic drainage in hypercholesterolemic mice may favor the accumulation of not only cholesterol, macromolecules, and fluid in tissues but also leukocytes, especially DCs. Previously, we showed in the same hypercholesterolemic mouse model that the migration of endogenous DCs from skin to draining lymph nodes was markedly compromised and associated with poor priming.15 It was not then known whether the defect in DC migration that developed in response to hypercholesterolemia was linked to an autonomous change in DCs themselves or to modifications in DC environment. Here, we provide evidence that alterations in lymphatic vessel structure and function can, at least in part, account for decreased DC migration and compromised immune responses in apoE−/− mice.15,33,34,35,36 Moreover, our findings that the compromised DC migration in apoE−/− mice fed a chow diet was associated with functional initial lymphatic vessels but degenerated collecting vessels further support the important role of collecting vessels in controlling DC migration.18
Further study is needed to elucidate the underlying mechanisms of initial and collecting vessel modifications occurring in hypercholesterolemic apoE−/− mice, which in turn lead to dysfunctional lymphatic vessels. The leakiness of lymphatic vessels and the poor migration of DC via lymphatic vessels suggest a compromised structural integrity of the lymphatic vessels in apoE−/− mice. Hypercholesterolemia may interfere with the composition and the organization of junctions in initial and collecting vessels by altering the expression of adhesion molecules and/or tight junction-associated proteins including VE-cadherin, claudin-5, and occludin.37 That oxidized low-density lipoprotein induces tight junction disassembly in aortic endothelia cells in vitro by decreasing occludin expression supports this possibility.38 Reductions in nitric oxide play a significant role in arterial dysfunction induced by hypercholesterolemia.17 Decreased nitric oxide may promote lymphatic dysfunction, but the mechanisms pertinent to lymphatic vessels are probably more complex than those in arteries, because lymphatic smooth muscle behaves more like cardiac muscle than like arterial smooth muscle,39 and the regulation of cardiac muscle function by nitric oxide is complex and can both positively and negatively affect contractile function.40 Although the abnormalities in lymphatic vessels may be directly triggered by hypercholesterolemia as a result of apoE deficiency, other mechanisms may also apply. Besides compromised clearance of lipoprotein, apoE−/− mice exhibit systemic inflammatory changes in many tissues, especially skin.12,14,15 Because increasing evidence in the literature suggests that inflammation has an impact on lymphatic vessels,18,41,42,43 inflammation in apoE−/− mice may account for the degeneration in the lymphatic vessels.
Normally, initial lymphatic vessels undergo intrinsic remodeling to mature into collecting vessels. This remodeling includes the down-regulation of the initial lymphatic marker LYVE-1, the coverage of collecting vessels with smooth muscle cells that are absent on initial lymphatics, and the formation of valves.44,45,46,47 During development, contact of lymphatic endothelium with smooth muscle cells has been shown to be critical for the down-regulation of LYVE-1 expression in initial lymphatic vessels during their maturation into collecting vessels and for the formation of intraluminal valves,45 but it is not known whether degeneration of smooth muscle coverage can lead to the up-regulation of LYVE-1 in collecting vessels in adulthood or whether valve structure can degenerate. Our findings suggest that this is indeed the case, because valves appeared to be malformed and LYVE-1 expression was high in the collecting vessels of older apoE−/− mice, whereas LYVE-1 expression was low in young apoE−/− mice with apparently normal valves and smooth muscle coverage. This result indicates that collecting vessels can in fact degenerate in the adult and lose these features. Because collecting vessels in hypercholesterolemic apoE−/− mice have little or no coverage with smooth muscle cells, these defects probably result from reduced interaction of lymphatic endothelium with smooth muscle cells. The paucity in smooth muscle cells around collecting vessels may result from their death by apoptosis, their detachment from lymphatic endothelium, or, alternatively, impaired migration. Foxc2, vascular endothelial growth factor receptor-3, and angiopoietin-2/Tie-2 signaling pathways have been recently described to control the recruitment of smooth muscle cells specifically to lymphatic vessels,44 and thus the environment in apoE−/− mice may interfere with these factors to affect collecting vessels.
Altogether, the results of this study provide evidence that hypercholesterolemic mice exhibit a notably lymphatic vessel remodeling in adult tissue, which in turn alters lymphatic transport of macromolecules, fluid, and immune cells. These findings point to the need for increased research on the mechanisms that link lymphatic vessels with lipid-related disorders and raise the possibility of a causative role of dyslipidemia in promoting alterations in lymphatic vessels reported in chronic inflammatory disorders that are associated with dyslipidemia, such as rheumatoid arthritis,48 psoriasis,49 and certain cancers.11
Supplementary Material
Acknowledgments
We thank Chung H. Thiam for cholesterol analyses.
Footnotes
Address reprint requests to Dr. Véronique Angeli, Department of Microbiology, Immunology Programme, National University of Singapore, Centre for Life Sciences, 03-05, 28 Medical Dr., Singapore 117456. E-mail: micva@nus.edu.sg.
Supported in part by the National Institutes of Health (grant HL075217 to M.S.S. and grants AI601741 and HL084312 to G.J.R.), by the Swiss National Science Foundation (107602 to M.A.S.), and by a Scientist Development Grant from the American Heart Association and a British Medical Research Council grant (to V.A.). G.J.R. is an Established Investigator of the American Heart Association (0720052N).
G.J.R. and V.A. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Nanjee MN, Cooke CJ, Wong JS, Hamilton RL, Olszewski WL, Miller NE. Composition and ultrastructure of size subclasses of normal human peripheral lymph lipoproteins: quantification of cholesterol uptake by HDL in tissue fluids. J Lipid Res. 2001;42:639–648. [PubMed] [Google Scholar]
- Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- Schirger A, Harrison E, Janes J. Idiopathic lymphedema. Review of 131 cases. JAMA. 1962;182:14–22. doi: 10.1001/jama.1962.03050400016004. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia. VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72:161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco V, Schwartz RA, Ruocco E. Lymphedema: an immunologically vulnerable site for development of neoplasms. J Am Acad Dermatol. 2002;47:124–127. doi: 10.1067/mjd.2002.120909. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Mortimer PS. Causes and clinical manifestations of lymphatic failure. Clin Dermatol. 1995;13:459–471. doi: 10.1016/0738-081x(95)00096-x. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Amann-Vesti BR, Franzeck UK, Bollinger A. Microlymphatic aneurysms in patients with lipedema. Lymphology. 2001;34:170–175. [PubMed] [Google Scholar]
- Bilancini S, Lucchi M, Tucci S, Eleuteri P. Functional lymphatic alterations in patients suffering from lipedema. Angiology. 1995;46:333–339. doi: 10.1177/000331979504600408. [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer JM. High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmun Rev. 2002;1:111–117. doi: 10.1016/s1568-9972(01)00018-0. [DOI] [PubMed] [Google Scholar]
- Sako A, Kitayama J, Kaisaki S, Nagawa H. Hyperlipidemia is a risk factor for lymphatic metastasis in superficial esophageal carcinoma. Cancer Lett. 2004;208:43–49. doi: 10.1016/j.canlet.2003.11.010. [DOI] [PubMed] [Google Scholar]
- van Ree JH, Gijbels MJ, van den Broek WJ, Hofker MH, Havekes LM. Atypical xanthomatosis in apolipoprotein E-deficient mice after cholesterol feeding. Atherosclerosis. 1995;112:237–243. doi: 10.1016/0021-9150(94)05419-j. [DOI] [PubMed] [Google Scholar]
- Zabalawi M, Bharadwaj M, Horton H, Cline M, Willingham M, Thomas MJ, Sorci-Thomas MG. Inflammation and skin cholesterol in LDLr−/−, apoA-I−/− mice: link between cholesterol homeostasis and self-tolerance? J Lipid Res. 2007;48:52–65. doi: 10.1194/jlr.M600370-JLR200. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Elias PM, Mao-Qiang M, Fartasch M, Zhang SH, Maeda N. Apolipoprotein E deficiency leads to cutaneous foam cell formation in mice. J Invest Dermatol. 1995;104:246–250. doi: 10.1111/1523-1747.ep12612790. [DOI] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Kamiya T, Yamamura T, Yokoyama S, Horiguchi Y, Funahashi T, Kawaguchi A, Miyake Y, Beppu S, Ishikawa K, Matsuzawa Y, Takaichi S. Clinical features of familial hypercholesterolemia. Arteriosclerosis. 1989;9:I66–74. [PubMed] [Google Scholar]
- Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dellinger MT, Hunter RJ, Bernas MJ, Witte MH, Erickson RP. Chy-3 mice are Vegfc haploinsufficient and exhibit defective dermal superficial to deep lymphatic transition and dermal lymphatic hypoplasia. Dev Dyn. 2007;236:2346–2355. doi: 10.1002/dvdy.21208. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA, Berk DA, Jain RK. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996;270:H324–H329. doi: 10.1152/ajpheart.1996.270.1.H324. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Kaipainen A, Netti PA, Brekken C, Boucher Y, Grodzinsky AJ, Jain RK. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech. 1999;32:1297–1307. doi: 10.1016/s0021-9290(99)00125-6. [DOI] [PubMed] [Google Scholar]
- Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Lin RY, Choudhury RP, Cai W, Lu M, Fallon JT, Fisher EA, Vlassara H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2003;168:213–220. doi: 10.1016/s0021-9150(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Osako MK, Takami Y, Hanayama R, Koriyama H, Mori M, Hayashi H, Shimizu H, Morishita R. Vascular protective effects of ezetimibe in ApoE-deficient mice. Atherosclerosis. 2009;203:51–58. doi: 10.1016/j.atherosclerosis.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Sawane M, Huggenberger R, Detmar M. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol. 2009;129:1292–1298. doi: 10.1038/jid.2008.351. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Cooke CJ, Nanjee MN, Stepanova IP, Olszewski WL, Miller NE. Variations in lipid and apolipoprotein concentrations in human leg lymph: effects of posture and physical exercise. Atherosclerosis. 2004;173:39–45. doi: 10.1016/j.atherosclerosis.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Jaggi M, Dumrese T, Brduscha-Riem K, Odermatt B, Hengartner H, Zinkernagel RM. Hypercholesterolemia exacerbates virus-induced immunopathologic liver disease via suppression of antiviral cytotoxic T cell responses. J Immunol. 2001;166:3369–3376. doi: 10.4049/jimmunol.166.5.3369. [DOI] [PubMed] [Google Scholar]
- de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- Netea MG, Demacker PN, de Bont N, Boerman OC, Stalenhoef AF, van der Meer JW, Kullberg BJ. Hyperlipoproteinemia enhances susceptibility to acute disseminated Candida albicans infection in low-density-lipoprotein-receptor-deficient mice. Infect Immun. 1997;65:2663–2667. doi: 10.1128/iai.65.7.2663-2667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowitz DT, Lee DM, Schmechel D, Staats HF. Altered immune responses in apolipoprotein E-deficient mice. J Lipid Res. 2000;41:613–620. [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaio L, Rouhanizadeh M, Reddy S, Sevanian A, Hwang J, Hsiai TK. Oxidized phospholipids mediate occludin expression and phosphorylation in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290:H674–H683. doi: 10.1152/ajpheart.00554.2005. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann NY Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- Paulus WJ, Frantz S, Kelly RA. Nitric oxide and cardiac contractility in human heart failure: time for reappraisal. Circulation. 2001;104:2260–2262. [PubMed] [Google Scholar]
- Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- Burman A, Haworth O, Hardie DL, Amft EN, Siewert C, Jackson DG, Salmon M, Buckley CD. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693–1700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff S, Bedlow AJ, Stanton AW, Mortimer PS. An in vivo study of the microlymphatics in psoriasis using fluorescence microlymphography. Br J Dermatol. 1999;140:61–66. doi: 10.1046/j.1365-2133.1999.02608.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.