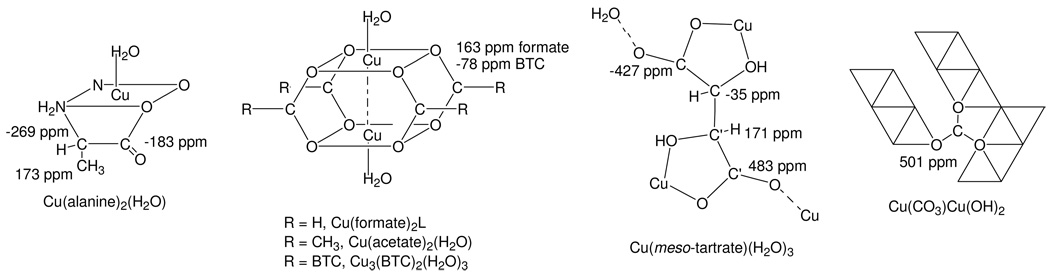
Figure 8.
Succinct structural elements of Cu3(BTC)2 and Cu(II)-containing model compounds indicating 13C MAS NMR assignments. Assignments may be reversed for Cu(tartrate)(H2O)3 (see text). Although the structure for Cu(meso-tartrate)(H2O)3 is shown, Cu(d-tartrate)(H2O)3 (and presumably the l-tartrate model compound under study) similarly possesses the two types of CO2− and HCOH groups which are differentiated by prime notation[29]. The bi-nuclear Cu(formate)2 core-structure shown is stabilized in the presence of amine-type ligands, but not water[30]. For Cu(CO)3Cu(OH)2 edge-sharing Cu(II) octahedral chains (two octahedrals wide) are represented by triangles, strips of which are linked[31] by the CO32− group as indicated.