Abstract
Objective
To evaluate the association of early pregnancy concentrations of thrombin-anthithrombin III complex with subsequent spontaneous preterm birth.
Methods
In a nested case-control study, thrombin-anthithrombin III complex was measured in plasma before 20 weeks of gestation (mean 9.9 weeks) among women without chronic conditions, preeclampsia or growth restriction. C-reactive protein (CRP) and non-HDL cholesterol were also measured. Women with spontaneous preterm birth before 34 weeks of gestation (n=29) and 34 to 36 weeks of gestation (n=72) were compared to women with term births occurring at or after 37 weeks (n=219). Polycotomous logistic regression was used to relate elevated thrombin-anthithrombin III complex (greater than 5.5 ng/ml), dyslipidemia (non-HDL cholesterol greater than the 90th percentile) and inflammation (CRP at or above 8ug/ml) to risk of spontaneous preterm birth subtypes.
Results
Women with spontaneous preterm birth vs. term births had elevated thrombin-anthithrombin III complex (p=0.02) and they were more likely to have a thrombin-anthithrombin III complex greater than 5.5 ng/mL (p<0.01). Women with thrombin-anthithrombin III complex in the highest vs. lowest quartile had a 4.6-fold (95% CI: 1.3-15.8) increased risk for spontaneous preterm birth before 34 weeks of gestation, adjusted for body mass index, race, inflammation, dyslipidemia, and gestational age at sampling. There was a dose response trend between thrombin-anthithrombin III complex and spontaneous preterm birth before 34 weeks (p<0.01) and 34 to 36 weeks (p=0.03).
Conclusions
There is evidence of early pregnancy systemic fibrinolysis among women with spontaneous preterm birth before 34 weeks of gestation independent of inflammation and dyslipidemia, perhaps secondary to microvascular injury.
Preterm birth affects 12.5% of births in the U.S.(1) It is the leading cause of perinatal morbidity and mortality, and of particular concern, the rate of singleton preterm births is increasing. Despite the profound health consequences for infants, preterm birth was not thought to be related to subsequent maternal morbidity until recently. Epidemiologic evidence indicates that women who have delivered a preterm birth have a 2 to 3-fold higher risk for cardiovascular disease (CVD) death compared to those with term births.(2-4)
Mechanisms that may link preterm birth with excess maternal CVD risk have not been studied, but we hypothesized that thrombosis/hemostasis factors may be involved. Shallow trophoblastic invasion has been associated with a third of spontaneous preterm births,(5, 6) and although the pathophysiology linking these vascular lesions to preterm birth is unclear, thrombin is thought to play a role. Plasma concentrations of thrombin-anthithrombin III complex (TAT), a sensitive marker of the coagulation cascade(7), are elevated in the second trimester among women with subsequent preterm birth.(8, 9) Activation of the fibrinolytic cascade may be related both to subclinical decidual hemorrhage, and either systemic or placental microvascular injury. To our knowledge, it is unknown if TAT concentrations are elevated early in normal or complicated pregnancies. It is certainly plausible that activation of fibrinolysis among women may be related to inflammation and dyslipidemia, given the relation of these factors to sPTB risk(10-13) as well as later life CVD risk in women.(14)
Our objective was to evaluate the association of early pregnancy concentrations of TAT with subsequent spontaneous preterm birth (sPTB). We also evaluated if early pregnancy TAT concentrations were related to systemic inflammation, as represented by C-reactive protein, and dyslipidemia, as represented by non-HDL cholesterol.
Material and Methods
The Pregnancy Exposures and Preeclampsia Prevention (PEPP) study was a prospective study of women enrolled <16 weeks gestation and followed through the post partum visit. Women were recruited from clinics and private practices from 1997 to 2001. The study was approved by the University of Pittsburgh institutional review board, and all participants provided written informed consent. Of the 2211 women enrolled, we excluded women with preexisting hypertension or diabetes, preeclampsia, transient hypertension, multiple gestation or positive toxicology screen. We also limited the analysis to the first birth in the cohort and to those with complete diagnostic information. Of the 1563 eligible women with otherwise uncomplicated pregnancies, 116 delivered preterm (before 37 weeks gestation). Cases were all women from this group with spontaneous preterm birth who had a first blood specimen drawn <20 weeks gestation (n=101). Controls (2:1) were randomly selected from women with uncomplicated pregnancies who delivered ≥ 37 weeks gestation with a blood sample collected <20 weeks. (n=219).
Maternal non-fasting plasma and serum samples were collected at the first prenatal visit and were stored in aliquots at -80° C until assayed. The first specimen drawn before 20 weeks gestation (mean 9.9 weeks, SD 3.6) was evaluated. All samples were analyzed after a maximum of three thaws with prior testing that results were stable. Plasma concentrations of TAT were measured using commercially available antibodies (Affinity Biologicals Inc.). ELISA was employed and all samples were run in duplicate with a correlation coefficient of 97.5%. Intra-assay coefficients of variation were 4-6% and inter-assay variation was 6-9%. The limit of detection was 2ng/mL. The individuals performing the ELISA were blinded to the case allocation of the plasma samples. TAT was characterized in quartiles based on the distribution among women with term births. TAT was also defined as a dichotomous variable in which women with concentrations in the highest quartile (>5.5 ng/mL) were compared to those with values below this threshold.
CRP was measured by high-sensitivity ELISA. The detection limit of the CRP assay was 0.2 ug/ml, with intra- and inter-assay variabilities of 4 percent and 7 percent, respectively. Inflammation was defined as CRP ≥8 ug/ml, as this threshold has been associated with chorioamnionitis (15) and has been previously related to preterm birth risk.(16) Total cholesterol and HDL cholesterol were measured in duplicate by a colorometric technique using commercial kits from Pointe Scientific (Canton, MI); the average coefficients of variation between runs ranged from 5.3 percent to 8.4 percent. Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol and has been used to relate atherogenic lipid components to CVD risk.(17) Previous reports have related both high and low cholesterol concentrations to preterm birth risk(13, 18) so we mirrored this approach and defined low non-HDL cholesterol as <10th percentile and high non-HDL cholesterol as >90th percentile among women with term births; concentrations between the 10th and 90th were the referent.
Gestational age was assessed upon delivery based on best obstetrical estimate. Women were categorized as delivering at 37 weeks or greater, at 34-36 weeks, and before 34 weeks gestation to describe severity of preterm status, as inflammation have been more strongly related to early preterm birth.(19) Spontaneous preterm births were defined as those occurring after spontaneous onset of preterm labor with intact membranes or following preterm spontaneous premature rupture of the fetal membranes.
Women enrolled in PEPP underwent a structured interview conducted by research staff at their first prenatal visit. Reported pre-pregnancy weight and measured height were used to calculate pre-pregnancy BMI (kg/m2). Self-reported covariates considered were maternal age at delivery, education (less than high school for women older than 19 who did not complete high school vs. high school or greater), periconceptional multivitamin use (self-reported multivitamin use during the 6 months prior to the first prenatal visit), smoking during pregnancy (any smoking since suspected pregnancy), and race. Due to the small number of women who reported their race as other than black or white (n=4), results are reported for black women vs. non-black women (white and other). Women reported if they were born small (< 2500 g), or if a mother or sister had a pregnancy complicated by preeclampsia or transient hypertension. Women reported any leisure physical activity in the year before they became pregnant, and the intensity was reported as low, moderate, or vigorous. Women were categorized as those who reported moderate or vigorous physical activity vs. low intensity or none.
Maternal characteristics were summarized according to sPTB status (≥37 weeks, 34 to 36 weeks, <34 weeks). TAT and CRP were not normally distributed (after examining plots and testing with the Shapiro-Wilk statistic (20) so median concentrations were compared using the Kruskal Wallis test and correlations were evaluated using Spearman correlation coefficients. Differences were considered significant with p < 0.05 and all tests were two tailed. Multivariable polycotomous logistic regression which allows for outcomes with more than two levels (21) was used to estimate the risk of sPTB (34 to 36 weeks and <34 weeks) associated with having early pregnancy elevated TAT. A second early pregnancy model then evaluated the joint effects of TAT, inflammation and dyslipidemia. Covariates were considered confounders if they changed the odds ratio associated with early pregnancy elevated TAT, CRP or non-HDL cholesterol by >10 percent. All covariates were evaluated in this fashion, and those that met our change in estimate criteria were included in the final model. All models were additionally adjusted for gestational age at sampling because TAT increases with gestation.(22) Likelihood ratio tests (p<0.10) were used to determine if the effect of TAT on sPTB risk was modified by either dyslipidemia or inflammation. (23) Linear trend was evaluated by adding an ordinal variable for increasing quartiles of TAT and by adding TAT to the models as a continuous variable. (23)
Results
Women with sPTB <34 weeks were more likely to be Black and to have less than a high school education compared to women with term births (Table 1). They were less likely to report periconceptional vitamin use and to have engaged in moderate or vigorous physical activity before pregnancy. They were also 3 to 4 times more likely to report that a mother or sister had pregnancies complicated by hypertension or that they themselves were small at birth.
Table 1.
Maternal characteristics according to preterm birth status, mean (SD) or percent, Pregnancy Exposures and Preeclampsia Prevention Study, 1997–2001
| ≥37 weeks (n=219) |
≥34 to <37 weeks (n=72) |
<34 weeks (n=29) |
|
|---|---|---|---|
| Demographic and lifestyle | |||
| Age (years) | 24.9 (6.0) | 24.9 (5.5) | 25.0 (6.7) |
| Black (%) | 31.5 | 34.7 | 51.7 |
| Less than high school education (%) * | 8.2 | 5.6 | 20.7 |
| Pre-pregnancy moderate or vigorous physical activity (%) | 32.4 | 26.4 | 13.8 |
| BMI (kg/m2) | 26.7 (6.6) | 25.7 (5.9) | 26.6 (6.8) |
| Family history | |||
| Family history preeclampsia or hypertension during pregnancy (%) * | 6.4 | 2.8 | 24.1 |
| Mother was <2500 g at birth (%) †* | 4.3 | 3.3 | 20.0 |
| Pregnancy | |||
| Nulliparous (%) | 61.2 | 48.6 | 51.7 |
| Periconceptional multivitamin use (%) | 46.6 | 48.6 | 24.1 |
| Smoking during pregnancy (%) | 31.5 | 34.7 | 34.5 |
| Gestational age at interview (weeks) | 9.7 (3.4) | 9.9 (3.7) | 10.7 (4.6) |
p<0.05 based on chi square or ANOVA
Available for 281 women
Women with TAT concentrations in the highest quartile (>5.5 ng/mL) vs. those with concentrations below this threshold were more likely to report that they were small at birth (9.6% vs. 3.6%, p=0.05) and they were somewhat less likely to be overweight prior to pregnancy (40.0% vs. 50.0%, p=0.10). There were no other meaningful differences among women with and without elevated TAT. Early pregnancy TAT concentrations were not linearly related with CRP (Spearman r=-0.04, p=0.42) or non-HDL cholesterol (Spearman r=-0.06;,p=0.29). Among overweight women (BMI >25 kg/m2), however, TAT was related to non-HDL cholesterol (Spearman r=0.25, p<0.01) but not to CRP.
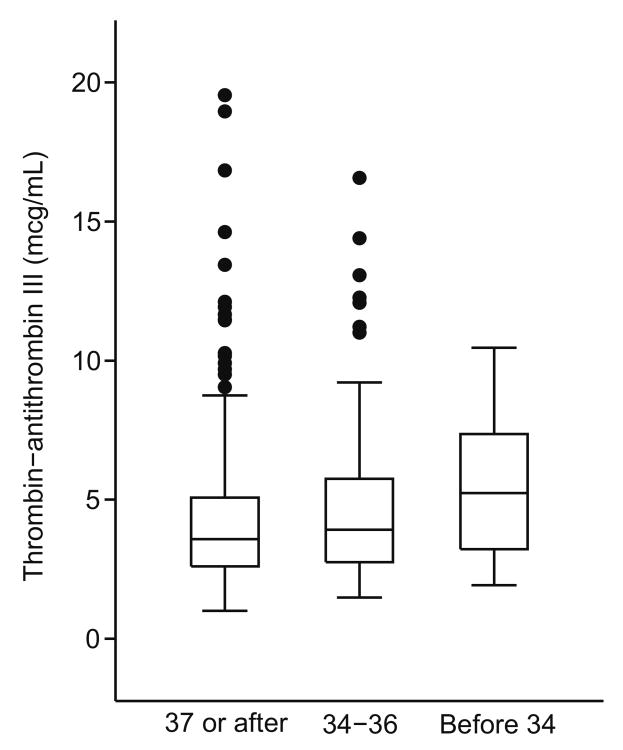
Median concentrations of TAT were higher among women with sPTB <34 weeks compared to those with sPTB 34-36 weeks and term births (<34 weeks, 5.6 ng/mL [Interquartile Range 2.0-7.6]; 34-36 weeks, 4.0 [Interquartile range 2.8-6.9; Term, 3.6 [Interquartile range 2.6-5.3]; p=0.02; Figure 1). Similarly, those with sPTB <34 weeks were more likely to have TAT concentrations in the highest quartile (>5.5 ng/mL) compared to those with moderate sPTB or term births (<34 weeks, 51.7%; 34-36 weeks, 29.2%; ≥ 37 weeks 22.8%, p<0.01).
Figure 1.
Distribution of thrombin-antithrombin III complex (TAT) before 20 weeks gestation, according to preterm birth status. The boxes represent the interquartile range (IQR) for each group and the circles represent the values >1.5 times the IQR. The horizontal line in each box represents the median concentration of TAT. Results were truncated at TAT concentrations of 20 ng/mL (6 observations excluded).
Women with TAT concentrations in the highest vs. the lowest quartile had a 4.2-fold (95% CI 1.2, 14.2) increased risk for sPTB <34 weeks after adjustment for maternal age, race, body mass index and gestational age at sampling (Table 2). In addition, risk of sPTB <34 weeks increased in a linear fashion as TAT concentrations increased (p for trend <0.01). Additional adjustment for early pregnancy inflammation or dyslipidemia did not affect these results. Indeed, both elevated TAT and elevated non-HDL cholesterol were each independently associated with about a 4-fold increased risk for sPTB <34 weeks. These results were attenuated but still significant when limited to samples collected before 15 weeks gestation (mean 8.8 weeks). For example, elevated TAT was associated with a 3.7-fold (95% CI 1.1, 12.9) increased risk for sPTB <34 weeks, adjusted for inflammation, dyslipidemia and other covariates.
Table 2.
Relation of TAT, CRP and non-HDL cholesterol to sPTB risk
| sPTB 34-<37 weeks (n=72) |
p trend | sPTB <34 weeks (n=29) |
p trend | |
|---|---|---|---|---|
| Model 1* | ||||
| TAT 4th quartile (>5.5 ng/mL) | 1.5 (0.7, 3.3) | 0.17 | 4.2 (1.2, 14.2) | <0.01 |
| TAT 3rd quartile (3.6-5.5) | 1.6 (0.8, 3.5) | 1.4 (0.3, 5.7) | ||
| TAT 2nd quartile (2.6-3.5) | 0.9 (0.4, 2.1) | 1.0 (0.2, 4.5) | ||
| TAT 1st quartile (<2.6) | 1.0 | 1.0 | ||
| Model 2† | ||||
| TAT 4th quartile (>5.5 ng/mL) | 1.7 (0.7, 3.7) | 0.10 | 4.6 (1.3, 15.8) | <0.01 |
| TAT 3rd quartile (3.6-5.5) | 1.8 (0.8, 3.9) | 1.5 (0.4, 6.2) | ||
| TAT 2nd quartile (2.6-3.5) | 0.9 (0.4, 2.2) | 1.06 (0.2, 4.8) | ||
| CRP >8 ug/ml | 2.9 (1.3, 6.2) | 2.3 (0.8, 6.7) | ||
| non-HDL cholesterol >180 mg/dl | 1.7 (0.6, 4.5) | 3.7 (1.1, 12.6) |
Model 1 adjusted for maternal age, race, body mass index and gestational age at sampling
Model 2 adjusted for maternal age, race, body mass index, non-HDL cholesterol <10th percentile, and gestational age at sampling. Women with TAT<2.6 ng/mL, CRP <=8 ug/ml and non-HDL cholesterol between 10th and 90th percentile are the referent
Increasing TAT quartiles were not related to increased risk for sPTB 34-36 weeks (p for trend=0.10). When modeled as a continuous variable, however, each one unit increase in TAT was associated with an 8% increased risk for moderate sPTB (OR 1.08, 95% CI 1.01, 1.16, p=0.03) suggesting a dose dependent relationship between TAT and sPTB 34-36 weeks. In addition, elevated CRP was associated with a 2.9-fold (95% CI 1.3, 6.2) increased risk for sPTB 34-36 weeks. There was no evidence that the relation between TAT and either sPTB subtype was different among women with elevated CRP or non-HDL cholesterol. Similarly, there was no effect measure modification between TAT and non-HDL cholesterol among overweight women (BMI >25 kg/m2).
Discussion
Our findings indicate that there is activation of the fibrinolytic cascade, as represented by the thrombin antithrombin III complex, early in pregnancy among women with sPTB <34 weeks. This effect appeared to be linear and was independent of early pregnancy evidence of inflammation and dyslipidemia. In addition, each of these factors remained independently associated with subsequent sPTB risk. Our results suggest that several atherogenic factors present in the first half of pregnancy may be related to subsequent sPTB.
Previous reports have related elevated TAT in the second and third trimesters to sPTB risk, particularly cases associated with PPROM. This relation is thought to be due to subclinical decidual hemorrhage or microvascular injury that provokes myometrial contractility. Our data suggests that perhaps evidence of these processes is present much earlier in pregnancy.
Adverse pregnancy outcomes have, in general, been associated with elevations of markers of thrombin activation in the maternal circulation. Increased concentrations of TAT have been demonstrated in maternal plasma at the time of diagnosis of intrauterine growth restriction,(24) pre-eclampsia,(25) preterm labor,(8, 26) and PPROM.(9, 26) These studies all evaluated thrombin activation at the time of clinical presentation with a pregnancy complication, whereas our samples were collected among asymptomatic, low risk women at the first prenatal visit.
Current evidence supports the placenta as the primary source of TAT generation.(27) Mutoh et al(27) demonstrated higher concentrations of TAT in the uterine vein compared to the maternal plasma in pregnant subjects, implicating the uterus and placenta itself as the source of excess TAT generation. Tissue factor (TF) is abundant in the placenta, due in part to its synthesis in the cytotrophoblast and syncytiotrophoblast, and can lead to the generation of activated thrombin through the extrinsic pathway.(8) The trophoblastic synthesis of TF has been hypothesized to play a role in early placental vascular development(8) and trophoblasts have been demonstrated to increase their expression of the thrombin receptor (PAR-1 and 3) during early gestation.(28) Impaired placental development has been observed in TF-deficient mice.(29) Thus, elevated TAT in the first half of pregnancy could be related to placental dysfunction or bleeding that ultimately leads to sPTB, but this possibility warrants further study.
Our findings suggest that throbmotic/hemostatic factors, inflammation, and dyslipidemia are present in the first half of pregnancy in women at elevated risk for preterm birth. This is plausible given the emerging evidence that these three pathologic components of atherosclerosis are associated with coronary arterial disease later in life,(30) and that evidence of atherosclerotic processes are detectable by young adulthood.(31) Post partum studies of women with and without preterm birth are needed to determine if these atherogenic factors persist and are related to sPTB recurrence as well as later life maternal cardiovascular disease.
Our results should be considered in light of several limitations. Although we had a relatively larger number of sPTBs, we still had limited power to evaluate three way interactions between TAT, CRP and non-HDL cholesterol which may be relevant. We also were limited in our ability to adequately evaluate the independent effect of these markers on subtypes of sPTB and this warrants further study. For example, in our fully adjusted models elevated CRP was no longer significantly related to sPTB <34 weeks. We were unable to determine if this was due to a possible interaction with TAT and/or non-HDL cholesterol, or if we were underpowered to create precise estimates. Future longitudinal studies, including those with pre-pregnancy measurements, which can evaluate the temporal relation between these biomarkers are also needed. In addition, other measures of the fibrinolytic cascade are necessary. Given that the strongest predictor of preterm birth is a previous history of preterm birth, this would be an important variable to consider in our analysis. This information, however, was not available in our cohort. Strengths of our study included our ability to evaluate early pregnancy samples in a population of women with data collected within a standardized research protocol. We also had a reasonably large number of moderate and early sPTBs.
Our findings indicate that early pregnancy activation of the fibrinolytic cascade is present among women with subsequent sPTB <34 weeks, perhaps secondary to microvascular injury. There was evidence that the relation between TAT and both early and moderate sPTB risk increased in a linear, dose response fashion and that these effects were independent of inflammation and dyslipidemia. Furthermore, each of these atherogenic factors was independently related to sPTB risk and if these factors persist post partum they could be related to sPTB recurrence as well as later CVD risk.
Acknowledgments
Funding was provided by NIH-2P01-HD30367 (Preeclampsia Program Project), NIH-5M01-RR00056 (Magee-Womens Clinical Research Center), and the BIRCWH-K12HD043441-06.
Footnotes
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
Presented as a poster at the 2008 Society for Gynecologic Research meeting, San Diego CA March 25-29.
References
- 1.Births:Final Data for 2004. CDC; 2006. National Vital Statistics Reports. [Google Scholar]
- 2.Irgens H, Reisaeter L, Irgens L, Lie R. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort. BMJ. 2001;323(7323):1213–17. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith G, Pell J, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–6. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 4.Smith GD, Sterne J, Tynelius P, Lawlor DA, Rasmussen F. Birth Weight of Offspring and Subsequent Cardiovascular Mortality of the Parents. Epidemiology. 2005 July;16(4):563–9. doi: 10.1097/01.ede.0000164790.96316.c0. [DOI] [PubMed] [Google Scholar]
- 5.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal Placental Vasculopathy and Infection: Two Distinct Subgroups Among Patients With Preterm Labor and Preterm Ruptured Membranes. Am J Obstet Gynecol. 1993;168(2):585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 6.Germain A, Carvajal J, Sanchez M, Valenzuela G, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 7.Fareed J, Hoppensteadt DA, Leya F, Iqbal O, Wolf H, Bick R. Useful laboratory tests for studying thrombogenesis in acute cardiac syndromes. 1998:1845–53. [PubMed] [Google Scholar]
- 8.Elovitz MA, Baron J, Phillippe M, Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. American Journal of Obstetrics & Gynecology. 2001 Nov;185(5):1059–63. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 9.Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ, Rosen T, et al. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001 Oct;10(5):297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- 10.Romero Roberto, Espinoza Jimmy, Gonçalves LuísF, Kusanovic JP, Friel L, Hassan Sonia. The Role of Inflammation and Infection in Preterm Birth. Semin Reprod Med. 2007;(01):021–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 12.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and Dyslipidemia Related to Risk of Spontaneous Preterm Birth. American Journal of Epidemiology. 2007 September 30; doi: 10.1093/aje/kwm273. 2007:kwm273. [DOI] [PubMed] [Google Scholar]
- 13.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, et al. Adverse Birth Outcome Among Mothers With Low Serum Cholesterol. 2007:723–33. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 14.Ridker P, Hennekens C, Buring J, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. NEJM. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 15.Bek K, Nielson F, Qvist I. C-reactive protein (CRP) and pregnancy. An early indicator of chorioamnionitis. A review. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1990;35:29–33. doi: 10.1016/0028-2243(90)90139-r. [DOI] [PubMed] [Google Scholar]
- 16.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-Reactive Protein in Early Pregnancy and Preterm Delivery. Am J Epidemiol. 2005 December 1;162(11):1108–13. doi: 10.1093/aje/kwi323. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program. Third Report of the National Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002 [Google Scholar]
- 18.Catov JM, Bodnar L, Kip K, Hubel CA, Ness RB, Harger G, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;107:610e1–e7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg R, Hauth J, Andrews W. Mechanisms of disease: intrauterine infection and preterm delivery. NEJM. 2000;342(20):1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro S, Wilk M. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 21.Hosmer D, Lemeshow S. Applied Logistic Regression. Second. New York: John Wiley & sons, Inc; 2000. [Google Scholar]
- 22.Eichinger S, Weltermann A, Pilipp K, Hafner E, Kaider A, Kittl E, et al. Prospective evaluation of hemostatic system activation and thrombin potential in healthy pregnant women with and without factor V Leiden. Thromb Haemost. 1999;82:1232–6. [PubMed] [Google Scholar]
- 23.Kleinbaum D, Kupper L, Muller K, Nizam A. Applied Regression Analysis and Other Multivariable Methods. 3rd. Pacific Grove: Duxbury Press; 1998. [Google Scholar]
- 24.Bellart J, Gilabert R, Fontcuberta J, Carreras E, Miralles M, Cabero L. Coagulation and fibrinolysis parameters in normal pregnancy and in pregnancy complicated by intrauterine growth retardation. Am J Perinatol. 1998;15:81–5. doi: 10.1055/s-2007-993903. [DOI] [PubMed] [Google Scholar]
- 25.Cadroy Y, Grandjean H, Pichon J, Desprats R, Berrebi A, Fournie A. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. BJOG. 1993;100:416–20. doi: 10.1111/j.1471-0528.1993.tb15264.x. [DOI] [PubMed] [Google Scholar]
- 26.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim Y, Bujold E, Edwin S, et al. Activation of the coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11(26873) doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 27.Mutoh S, Kobayashi M, Hirata J, Itoh N, Maki M, Komatsu Y, et al. Studies on blood coagulation-fibrinolysis system regarding kallikrein-kinin system in the utero-placental circulation during normal pregnancy, labor and puerperium. Agents Actions Suppl. 1992;38:320–9. [PubMed] [Google Scholar]
- 28.Even-Ram S, Grisaru-Granovsky S, Pruss D, Maoz M, Salah Z, Yong-Jun Y, et al. The pattern of expression of protease-activated receptors (PARs) during early trophoblast development. J Pathol. 2003;200:47–52. doi: 10.1002/path.1338. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105:2777–82. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 30.Sagastagoitia JD, Saez Y, Vacas M, Narvaez I, Saez de Lafuente JP, Molinero E, et al. Association between inflammation, lipid and hemostatic factors in patients with stable angina. Thromb Res. 2007;120(1):53–9. doi: 10.1016/j.thromres.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, Iii, Herderick EE, et al. Prevalence and Extent of Atherosclerosis in Adolescents and Young Adults: Implications for Prevention From the Pathobiological Determinants of Atherosclerosis in Youth Study. 1999:727–35. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]