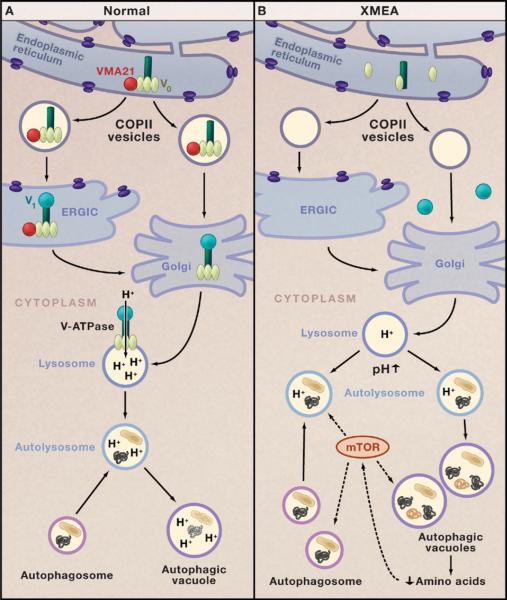
Figure 1. V-ATPase and Autophagy in Health and Disease.
(A) The chaperone VMA21 (red circle) binds to the c′ subunit of the vacuolar-ATPase (V-ATPase; dark green) in the endoplasmic reticulum and initiates assembly of the proton-translocator domain (V0; pale green) (Ramachandran et al., 2009). VMA21 chaperones V0 in COPII vesicles to the endoplasmic reticulum Golgi intermediate complex (ERGIC). VMA21 is not present in the Golgi apparatus, but it is here that V-ATPase assembly is completed by addition of the ATP-hydrolytic domain (V1; turquoise). Proton pumping by the V-ATPase is critical for regulating the pH of lysosomes. Fusion of lysosomes with autophagosomes generates autolysosomes that degrade target proteins and organelles.
(B) In the disease X-linked myopathy with excessive autophagy (XMEA), deficiency of VMA21 leads to failure of V0 assembly, reduced levels of V-ATPase, and a decrease in lysosomal pH that impairs lysosomal and autophagic digestion of cellular proteins and organelles. Decreased protein degradation in XMEA may lower intracellular levels of amino acids that modulate the mTOR signaling pathway, which, in turn, upregulates macroautophagy. This leads to excessive accumulation of autophagosomes, autolysosomes, and autophagic vacuoles and a muscle-specific disease phenotype.