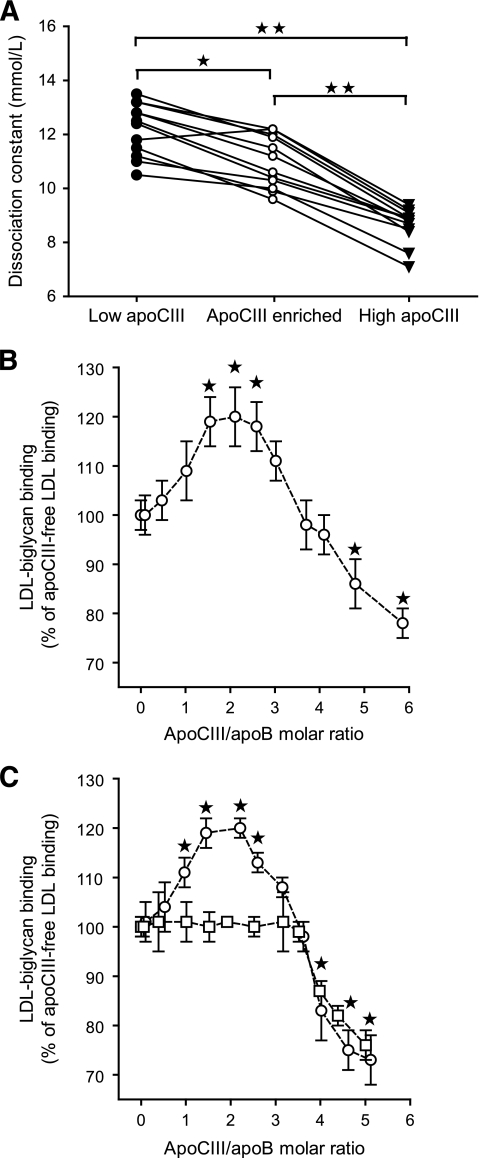
FIG. 1.
Effect of apoCIII content on binding of LDL to biglycan in vitro. A: The binding of LDL isolated from patients with type 2 diabetes was analyzed using solid-phase assays for biglycan in vitro. Twelve pairs of LDL with low (●) or high (▾) LDL-apoCIII were analyzed. The low– LDL-apoCIII samples were also enriched in vitro with human apoCIII (○) so they contained approximately as much apoCIII as the high LDL-apoCIII samples, and their binding was analyzed. The dissociation constants (Kd) of the corresponding samples in each matched pair are shown. *P = 0.005, **P < 0.001. B: LDL with no apoCIII was isolated from subjects with type 2 diabetes and enriched in vitro with increasing amounts of human purified apoCIII, and LDL binding was analyzed by solid-phase assays for biglycan. C: Recombinant wild-type LDL (○) and site A mutant LDL (□) were isolated from human apoB100 transgenic mice and enriched with increasing amounts of human purified apoCIII. The LDL binding was analyzed by solid-phase assays for biglycan and is expressed as a percentage of apoCIII-free LDL binding to biglycan (means ± SD, n = 4–5). *P < 0.05 versus apoCIII-free LDL.