FIG. 8.
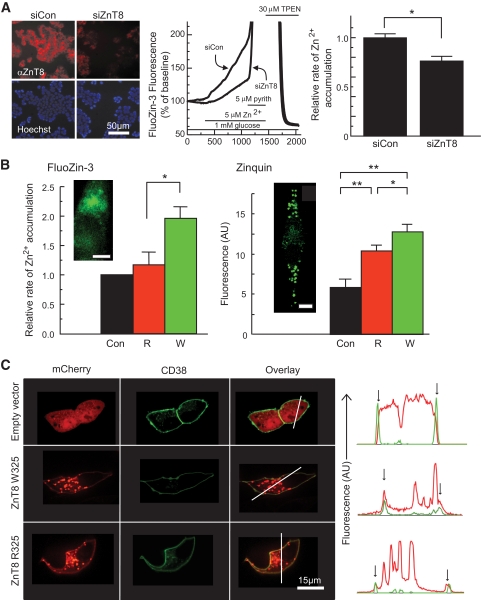
Role of ZnT8 in cellular Zn2+ transport and effects of the R325W polymorphism. A: ZnT8 expression was reduced by RNAi as assessed by immunocytochemical analysis of cells fixed in 4% (v/v) paraformaldehyde and incubated with rabbit anti-mouse ZnT8 antibody (Mellitech, 1:3,000) and anti-rabbit–conjugated secondary antibody (1:500) plus Hoechst dye (5 μg/ml) before imaging on a Zeiss Axiovert inverted optics microscope. Zn2+ uptake into single MIN6 cells was assessed by monitoring changes in FluoZin-3 fluorescence (research design and methods). Under the conditions used in these experiments, the FluoZin-3 fluorescence increase gives an almost linear readout during the perifusion with ZnSO4. The relative rates of Zn2+ accumulation were calculated from the slopes of the corresponding fluorescence changes. *P < 0.05 versus siCon, n = 5 independent experiments, 108–117 cells per condition. Calibrated resting free [Zn2+]i was 600–700 pmol/l (NS, higher vs. lower risk). B: R325 or W325 ZnT8 were overexpressed in MIN6 cells, and transfected cells were identified by fluorescence of cotransfected mCherry-expressing vector before measurements of the initial rate of apparent Zn2+ uptake as in A. *P < 0.05 versus control or ZnT8-R, n = 5 independent experiments, 67–115 cells per condition. Zinc accumulation into granules was assessed using zinquin after incubation for 4 h in the presence of 10 μmol/l ZnSO4. Insets show the localization in single cells of FluoZin-3 and zinquin, as monitored by confocal microscopy. Note the punctuate distribution of zinquin, consistent with accumulation of the dye into secretory granules; scale bar, 5 μm. (C) Localization of overexpressed ZnT8-mCherry chimaeras at the plasma membrane, identified using CD38-EGFP. Note the presence of plasma membrane–associated ZnT8-mCherry fluorescence (red), coincident with CD38-EGFP (green) in the line plots (vertical arrows). (A high-quality digital representation of this figure is available in the online issue.)