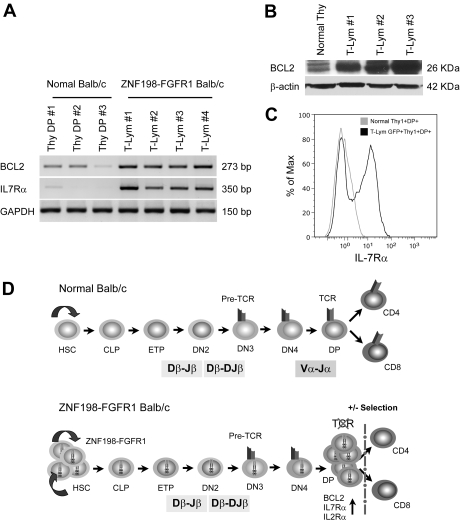
Figure 5.
Up-regulation of prosurvival genes protected immature T cells from ongoing apoptosis. (A) RT-PCR shows that transcription levels of Bcl2 and IL7R were remarkably up-regulated in T lymphomas compared with normal Thy1+CD4+CD8+ thymocytes. (B) High expression of BCL2 protein was also seen in T lymphoma versus that in normal BALB/c thymus by immunoblotting. (C) Flow cytometric analysis with anti-IL7Rα (CD127-PE) showing cell membrane IL7Rα expression levels in Thy1+DP lymphoma cells is approximately 10 times higher than that seen in normal Thy1+DP thymocytes. (D) A proposed mechanism for T-lineage lymphoblastic lymphoma development in the ZNF198-FGFR1 mouse model. The top panel from a normal mouse shows that early thymocyte progenitors (ETP) migrate from BM common lymphoid progenitors (CLP) into the thymus, and then sequentially differentiate into CD4−CD8− double-negative and CD4+CD8+ DP cells after Tcrb and Tcra rearrangements. Cells that express a TCR-CD3 complex can pass through both positive and negative selection and differentiate into CD4+, CD8+ mature T cells, or natural killer T cells (data not shown). The bottom panel proposes that self-renewal of hematopoietic stem cell (HSC) is accelerated when the ZNF198-FGFR1 fusion kinase is expressed. Concordantly, constitutive activation of high expression of pre-Tα promotes the progenitors to differentiate into DP cells, although loss of Tcra prevents immature T cells from forming a functional TCR-CD3 cell-surface complex, which results in arrested T-cell development at the DP stage. Furthermore, due to high expression of BCL2, IL2Rα, and IL7Rα, these DP cells can escape apoptosis and maintain high rates of cell proliferation.