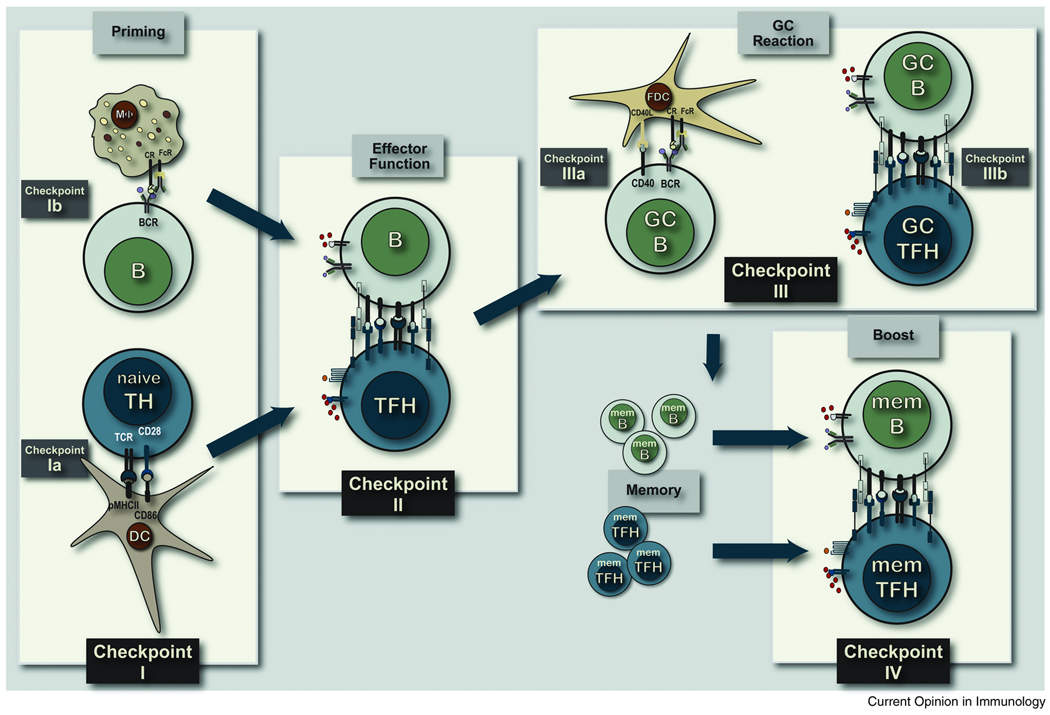
FIGURE 1. COGNATE TH CELL REGULATION OF B CELL IMMUNITY.
Following local protein vaccination, mature antigen-experienced dendritic cells from the site of injection traffic to the draining lymphoid tissue to prime pMHCII-specific naive TH cells at Checkpoint Ia. Antigen can also be transported to the subcapsullar sinus by macrophage to present native cell associate antigen to B cells at Checkpoint Ib. Antigen-specific B cells will take up protein antigen, process and present pMHCII complexes and move towards the T-B borders to interact with pMHCII-specific effector TFH cells at Checkpoint II. Following stable cognate contact a cohort of antigen-specific B cells will move into the follicular regions, massively expand to form secondary follicles, somatically diversify their BCR, express the variant BCR and then traverse FDC networks in the light zone of germinal center at continuous Checkpoint IIIa interactions. GC B cells expressing high affinity variant BCR form stable contacts with GC TFH cells at Checkpoint IIIb prior to GC exit and entry into the memory B cell compartment as either memory response precursors or long-lived plasma cells. Antigen-specific memory TFH cells and memory B cells persist in the priming lymphoid tissue to interact upon secondary challenge with antigen at Checkpoint IV a requisite regulatory interaction for expansion of memory B cells and formation of memory response plasma cells.