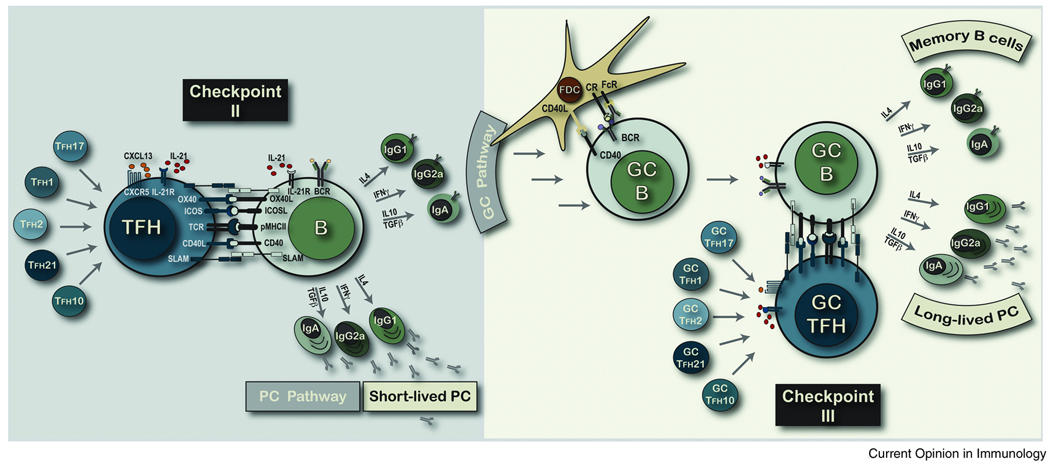
FIGURE 2. SEPARABLE ANTIGEN-SPECIFIC TFH AND B CELL LINEAGES.
We depict critical sets of molecular interactions at Checkpoint II that appear common to all antigen-specific effector TFH interactions with antigen-primed pMHCII-expressing B cells. We propose that all sub-types of functionally distinct TH cell lineages can also program the migration to the T-B borders and B cell zones to become TFH cells and regulate B cell fate and function. Further, these separate effector TFH lineages, depicted according to their original TH cell program, will promote distinct B cell lineage decisions at Checkpoint II. We propose that antibody isotype is a major ‘lineage’ decision for antigen-specific B cells and that within each isotype-specific lineage the secondary outcomes of plasma cell versus GC B cell fate may be controlled by the duration of contact at Checkpoint II. Entry into the GC pathway involves clonal expansion, somatic BCR diversification and affinity-based selection for all isotype-specific GC B cells at Checkpoint IIIa. GC B cells with a positive selection ‘imprint’ at Checkpoint IIIa will form stable cognate interactions with pMHCII-specific GC TFH cells at Checkpoint IIIb. We propose that there is some lineage maintenance between the effector TFH and the GC TFH creating distinct TFH lineages dedicated to the regulation of separate isotype-specific B cells. In this model, IL-4 expressing GC TFH will re-connect with IgG1+ GC B cells and IFN-γ+ TFH will re-connect with IgG2a+ GC B cells as demonstrated by Locksley and colleagues [40**]. It is plausible that the duration of intercellular contact regulates memory B cell versus long-lived plasma cell fate and function at this point of memory B lineage development.