Figure 3. Proneuropeptides: structural features for proteolytic processing.
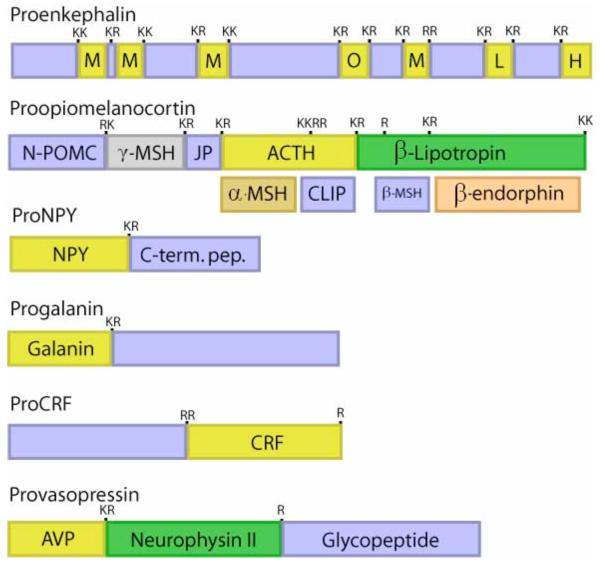
Neuropeptides are synthesized as proneuropeptide precursors, also known as prohormones, that require proteolytic processing to liberate the active neuropeptide. Proteolytic processing occurs at dibasic and monobasic sites, as well as at multibasic sites. The precursor proteins may contain one copy of the active neuropeptide, such as the proneuropeptides for NPY, galanin, CRF, and vasopressin. Some proneuropeptides such as proenkephalin contains multiple copies of the active neuropeptide; proenkephalin contains four copies of (Met)enkephalin (ME), one copy of (Leu)enkephalin (LE), and the related opioid peptides ME-Arg-Phe (H) and ME-Arg-Gly-Cleu (O). Certain precursors contain different peptide hormones within the same precursor, such as the POMC precursor which gives rise to the distinct peptide hormones ACTH, α-MSH, and ß-endorphin. The presence of ACTH in anterior pituitary, and the presence of α-MSH and ß-endorphin in intermediate pituitary illustrate that tissue-specific processing of the POMC prohormone occurs.
