Abstract
Little is known about neural responses in the early automatic-stage processing of rejection cues from a partner. Event-related potentials (ERPs) offer a window to study processes that may be difficult to detect via behavioral methods. We focused on the N400 ERP component, which reflects the amount of semantic processing prompted by a target. When participants were primed by attachment-related contexts (“If I need help from my partner, my partner will be …”), rejection-related words (e.g., dismissing) elicited greater N400 amplitudes than acceptance-related words (e.g., supporting). Analyses of results for nonattachment primes suggest that these findings were not simply caused by target valence; the brain responds differentially to cues of partner rejection versus acceptance in under 300 ms. Moreover, these early-stage neurophysiological responses were heightened or dampened as a function of individuals’ adult attachment; women characterized by high anxiety and low avoidance showed the greatest N400 responses to cues of partner rejection (vs. acceptance).
An enduring challenge for psychological science is to illuminate how people continuously monitor their environments for threats to their physical and psychological well-being. Given the importance of close relationships, an especially pernicious threat is rejection, real or imagined, by significant others. There have been important advances in understanding psychological and physiological responses to interpersonal rejection (e.g., Downey & Feldman, 1996; Downey, Mougios, Ayduk, London, & Shoda, 2004; Powers, Pietromonaco, Gunlicks, & Sayer, 2006). Studies have focused mostly on downstream processes, such as attributions (e.g., Mikulincer, 1998), appraisals (e.g., Mikulincer & Florian, 1998), and coping strategies (e.g., Collins & Feeney, 2000; Shaver & Hazan, 1993; Simpson, Rhodes, & Nelligan, 1992), speaking to how individuals might magnify or diminish initial automatic responses to threat cues. In contrast, little is known about neural responses in the early automatic-stage processing of partner rejection cues, which are implicated in the etiology and course of anxiety and mood disorders (e.g., Compton, 2003; Derryberry & Reed, 2002). With the aim of addressing this crucial gap, we used event-related potentials (ERPs) to assess normative responses in early-stage processing of partner rejection cues, and examined how these neural responses may be linked to individual differences in adult attachment.
People are highly sensitive to cues that may signal a threat to their most significant relationships (e.g., Bowlby, 1969). Although the system for monitoring such cues has adaptive functions (it triggers a cascade of psychological and physiological responses that operate to reduce the initial threat), high vigilance for potential threats comes at a price. It increases the frequency and intensity of destructive emotions, such as hostility, jealousy, and anxiety, as well as emotional and physiological distress (e.g., Ayduk, Downey, & Kim, 2001; Ayduk, Downey, Testa, Yen, & Shoda, 1999; Radecki-Bush, Farrell, & Bush, 1993).
Not surprisingly, researchers have hypothesized that individual differences in sensitivity to rejection cues play a critical role in insecure adult attachment (Hazan & Shaver, 1987; Mikulincer & Shaver, 2007). Previous work, relying mostly on behavioral measures and self-reports, has found that anxiously attached individuals show hypervigilance and sensitivity to rejection cues (e.g., Mikulincer, Birnbaum, Woddis, & Nachmias, 2000; Mikulincer, Gillath, & Shaver, 2002). They turn their attention toward threatening information and have a difficult time disengaging from threatening stimuli (e.g., Mikulincer & Orbach, 1995). Avoidantly attached individuals, in contrast, are characterized by interpersonal avoidance and defensiveness (e.g., Fraley & Shaver, 1997). They turn their attention away from threatening information and protect themselves through disengagement. A combination of high anxiety and low avoidance has been linked with difficulty in disengaging from threatening stimuli (Dewitte, Koster, De Houwer, & Buysse, 2007).
No research, however, has investigated how early the brain responds differentially to partner rejection cues, and how early individual differences in such differential neurophysiological responding begin to emerge. To address these issues, a temporally precise measure of processing is necessary. ERPs, sampled every 5 ms, are a noninvasive way to assess the electrical activity that emanates from the scalp and reflects the processing of events. The direction, onset, and duration of deflections in the ERP waveform meaningfully reflect particular cognitive, affective, and motor operations. Given their temporal precision, ERPs are particularly well suited for elucidating initial processing of partner rejection cues.
Past research using ERPs has demonstrated that the emotional value of generic stimuli (e.g., expressions of emotion by unfamiliar people or generic emotional images) can be encoded within 300 ms of their presentation (e.g., Pizzagalli, Regard, & Lehmann, 1999). Moreover, individual differences in adult attachment have been linked to ERP responses to generic, emotionally arousing pictures. Zilber, Goldstein, and Mikulincer (2007) found that higher attachment anxiety was associated with a larger late positive potential (LPP), a late deflection in the ERP waveform assumed to reflect the extent to which negative emotions are intensified after threatening aspects of a stimulus have been processed. The present research went beyond past work to investigate the processing of partner rejection cues and individual differences in such responses.
An ERP component that may be particularly useful in studying the processing of partner rejection cues is the N400, a negative-going deflection that starts at approximately 250 ms and peaks at approximately 400 ms after the onset of a target word (Kutas & Hillyard, 1980, 1984). N400 amplitude is associated with effort in retrieving a word’s meaning from the lexicon or in integrating semantic information into a mental representation (e.g., Hagoort, Hald, Bastiaansen, & Petersson, 2004; Kutas & Hillyard, 1980; Kutas, Van Petten, & Kluender, 2006). A critical characteristic of the N400 ERP component is that it is attuned specifically to semantic or conceptual analyses (e.g., Kutas & Hillyard, 1980, 1984). Moreover, target stimuli that are attended produce greater N400 amplitudes than nonattended stimuli (Bentin, Kutas, & Hillyard, 1995; Kiefer & Brendel, 2006). Thus, the most widely accepted view of the N400 is that it reflects the total amount of semantic processing elicited by a target, with more negative-going and longer-lasting N400 amplitudes indicating greater processing.
Our first goal in the present research was to assess participants' brain neurophysiological activity when they encountered partner rejection cues. We recorded ERPs while participants performed a lexical decision task (LDT; Baldwin, Fehr, Keedian, Seidel, & Thomson, 1993; Neely, 1977) in which to-be-classified words referred to partner rejection or acceptance. We predicted that partner rejection cues would elicit greater processing, reflected by more negative-going N400 amplitudes, than partner acceptance cues. Our second goal was to examine whether N400 amplitudes in response to rejection cues are modulated by women’s adult attachment. Do more anxiously attached women show a greater sensitivity to a partner's rejecting behaviors, as indexed by greater N400 amplitudes to partner rejection cues? Conversely, do more avoidantly attached women show less extensive processing of a partner's rejecting behaviors, as reflected by diminished N400 amplitudes to partner rejection cues?
METHOD
Participants
Thirty-five women (median age = 19 years, SD = 1.50 years) were preselected for participation if they had been involved in a romantic relationship for at least 6 months (median duration = 22 months, SD = 14.15 months). The research focused only on women, because past research has found that women respond more strongly than men to interpersonal events (e.g., Ayduk et al., 2001; Rudolph & Hammen, 1999).
Procedure and Measures
LDT
The LDT is a computerized task in which target stimuli are presented, one by one, in the middle of the computer screen. Participants’ task was to quickly and accurately classify targets as words or nonwords by pressing the "D" or "K" key on the keyboard, using their left or right forefinger, respectively. Assignment of keys to responses was counterbalanced across participants. On each trial, a fixation cross (+) appeared on the screen; approximately 200 ms later, an audio prime lasting approximately 3.2 s was presented; finally, approximately 16.7 ms after the end of the audio prime, the target stimulus appeared, replacing the fixation cross. The intertrial interval was 400 ms.
Prime stimuli were recorded by a female speaker and described 40 attachment-related contexts (“If I need help from my partner, my partner will be …”) and 40 nonattachment contexts (“If I deposit a check, my partner will be …”). The nonattachment primes described situations that were not expected to activate attachment-related needs, but were identical to the attachment primes in their syntactic structure, as well as in the fact that they used the first person to refer to the participant and the participant’s partner. Target stimuli consisted of 80 acceptance-related (e.g., supporting) and rejection-related (e.g., dismissing) words and 80 orthographically correct nonwords (e.g., kating). Each target was paired twice: once with an attachment prime and once with a nonattachment prime.
After 20 practice trials, participants completed four 80-trial blocks. Half of the trials involved nonword targets paired with either an attachment prime or a nonattachment prime. The remaining half of the trials involved word targets and consisted of four trial types (2 prime types × 2 target valences). Within each block, the six trial types appeared an equal number of times, randomly interspersed. The LDT was administered on IBM-compatible desktop computers with a Windows XP operating system using Inquisit Version 2.0 (Millisecond Software, Seattle, WA).
ERPs
Continuous electroencephalogram (EEG) was recorded from 20 channels using tin electrodes attached to an elastic cap (Electro-cap International, Eaton, OH). Electrodes were placed over the left and right prefrontal (Fp1, Fp2), frontal (F3, F4), inferior frontal (F7, F8), temporal (T7, T8), central (C3, C4), parietal (P3, P4), posterior parietal (P7, P8), and occipital (O1, O2) locations, and at three midline locations (Fz, Cz, Pz). Vertical and horizontal eye movements were recorded via electrodes placed below the left eye and to the right of the right eye, respectively. The 19 channels were referenced to an electrode placed over the left mastoid bone. Activity recorded over the right mastoid with a 20th channel was used to assess effects of experimental variables on the mastoid recordings. No such effects were observed. The EEG was amplified with a band pass of 0.01 through 100 Hz (3-dB cutoff) by an SAI bioamplifier system. The EEG and stimulus trigger codes were digitized on-line by a Data Translation 2801-A board using a sampling frequency of 200 Hz. Trials contaminated by excessive eye movement or muscle artifacts were excluded (11% in each prime condition).
Experiences in Close Relationships (ECR) Questionnaire
We assessed how participants typically experienced romantic relationships by having them complete the ECR (Brennan, Clark, & Shaver, 1998).1 The ECR consists of an 18-item attachment-avoidance scale, which assesses discomfort with intimacy and dependency, and an 18-item attachment- anxiety scale, which assesses vigilance concerning rejection and abandonment. Participants completed the ECR at the prescreening session (anxiety: α = .92; avoidance: α = .91) and again at the experimental session (anxiety: α = .95; avoidance: α = .92). Scores at the two assessments were moderately correlated with one another (anxiety: r = .42, p = .01; avoidance: r = .44, p = .008) and were similarly related to N400 and behavioral responses. Therefore, we used the means of the two assessments in subsequent analyses.
Data Reduction and Analyses
ERPs
ERPs, time-locked to the onset of the target, were averaged for each trial type. For the N400 analyses, we calculated mean amplitudes within the 250- to 600-ms window for each electrode position. Resulting values obtained at midline, medial-lateral, and lateral-lateral sites were analyzed within separate analyses of variance (ANOVAs). In all ANOVAs, prime type, target type, and electrode site (three midline electrodes: frontal, central, parietal; five medial-lateral electrodes: prefrontal, frontal, central, parietal, occipital; three lateral-lateral electrodes: inferior frontal, temporal, posterior parietal) were entered as within-subject factors. In addition, in ANOVAs involving medial-lateral and lateral-lateral sites, hemisphere was included as a within-subjects factor to test for hemispheric (left vs. right) differences. In cases in which the assumption of homogeneity of variance was violated, the Greenhouse-Geisser correction was applied, and corrected p values are reported.
LDT
For each trial in the LDT, response times (RTs; in milliseconds) and accuracy were recorded. Data from the first two trials of each block and from trials with RTs outside the normal range (< 150 ms or > 4,999 ms) were excluded from further analysis. RTs less than 300 ms and greater than 3,000 ms were recoded to 300 ms and 3,000 ms, respectively. All analyses were conducted on log-transformed RTs, but untransformed RTs (in milliseconds) are reported for illustrative purposes. Error rates for classifying word targets were low (M = 3.4%, SD = 0.03%) and showed no main effects of prime or target type, and no interaction effect.
RESULTS
ERP Data
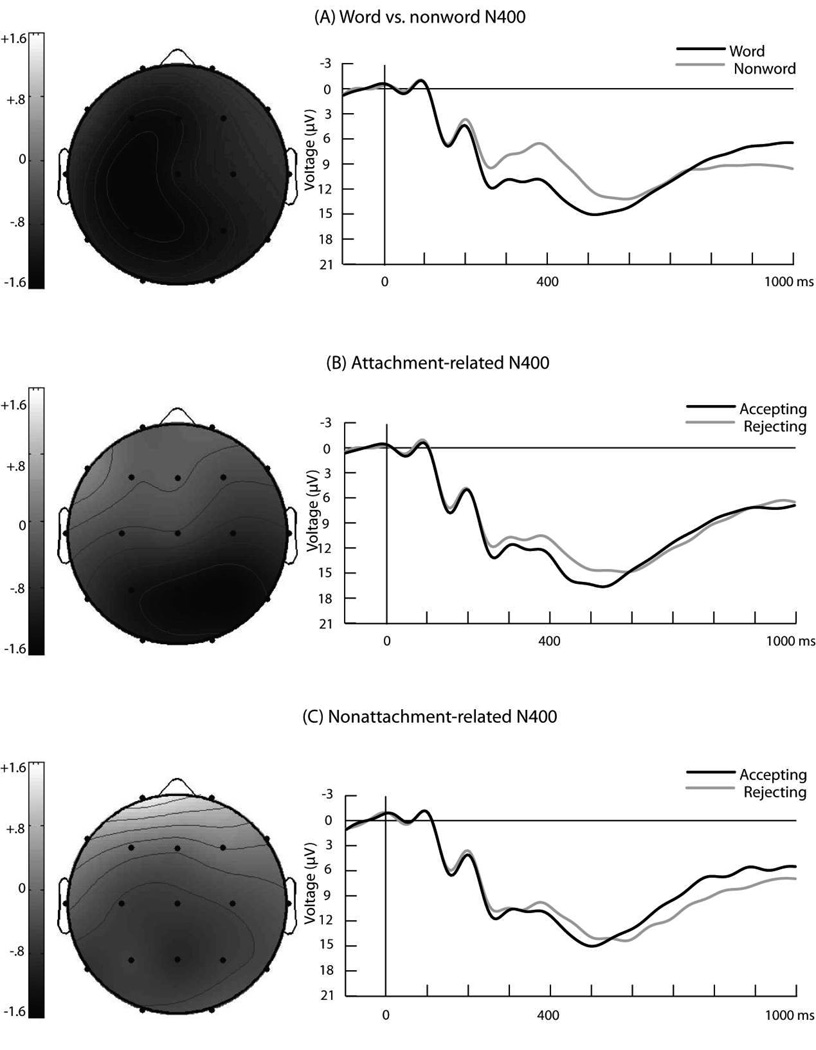
Our first set of ERP analyses was aimed at providing empirical support for the validity of the procedures used in this study. Past research (Kutas & Hillyard, 1984) has shown that orthographically correct nonwords elicit a more negative-going N400 than orthographically correct words, presumably because of the additional processing required to make sense of nonwords. We replicated this effect. Figure 1a shows the grand-average ERP waveforms recorded at P4 for nonword and word targets, as well as the distribution of the word-versus-nonword N400 effect throughout the scalp. ANOVAs showed that the N400 was more negative-going for nonword targets than for word targets at the midline, F(1, 34) = 45.14, p < 10−7, η2 = .57; at medial-lateral sites, F(1, 34) = 41.24, p < 10−6, η2 = .55; and at lateral-lateral sites, F(1, 34) = 39.88, p < 10−6, η2 = .54.
Fig. 1.
Grand-average event-related potential waveforms recorded at P4 and topographical maps showing the distribution of observed N400 effects. The graphs show the waveforms for (a) words and nonwords, (b) acceptance and rejection words after attachment-related primes, and (c) acceptance and rejection words after nonattachment primes. Negative voltage is plotted upward. The vertical line in each plot indicates the onset of the target stimulus. The topographical maps depict the voltage distribution, from 250 to 600 ms after target onset, of the following N400 effects (a) the word-versus-nonword effect, (b) the attachment-related effect (acceptance vs. rejection words), and (c) the nonattachment-related effect (acceptance vs. rejection words). Darker gray areas represent greater negative-going N400 amplitude for nonwords than words in (a), and greater negative-going amplitudes for rejection than for acceptance words in (b) and (c). The topographical maps do not specify the neural generators involved in the processing of targets.
Sensitivity of N400 Amplitude to the Processing of Partner Rejection Cues
Is the N400 amplitude sensitive to the processing of partner rejection cues? Figure 1b shows the grand-average ERP waveforms recorded at P4 for rejection and acceptance words, as well as the distribution of the attachment-related N400 effect (i.e., difference in N400 amplitude between rejection and acceptance words after attachment primes) throughout the scalp. After an attachment-related prime, rejection words elicited greater N400 amplitudes than acceptance words at the midline, F(1, 34) = 8.28, p = .007, η2 = .20; at medial-lateral sites, F(1, 34) = 7.20, p = .01, η2 = .18; and at lateral-lateral sites, F(1, 34) = 4.59, p = .04, η2 = .12. The attachment-related N400 effect was more pronounced in the right than in the left hemisphere, as reflected by a statistically significant Target Type (rejection vs. acceptance) × Hemisphere interaction at the medial-lateral sites, F(1, 34) = 5.85, p = .02, η2 = .15, and the lateral-lateral sites, F(1, 34) = 9.46, p = .004, η2 = .22. Tests of the linear effect indicated that the attachment-related N400 effect increased in a linear fashion from frontal to posterior sites of the midline, F(1, 34) = 5.80, p = .02, η2 = .15. The distribution of the attachment-related N400 effect across the scalp is consistent with past research showing that the posterior sites of the right hemisphere exhibit particularly large N400 effects (Kutas & Hillyard, 1980, 1982; Kutas, Van Petten, & Besson, 1988).
We tested whether the modulation of N400 amplitude by rejection words (relative to acceptance words) was greater after attachment-relevant primes than after nonattachment-relevant primes. Figure 1c shows the grand-average ERP waveforms recorded at P4 for rejection and acceptance word targets after a nonattachment-relevant prime, as well as the distribution of this difference across the scalp. This difference was smaller than the difference observed after the attachment-relevant primes. Specifically, ANOVAs showed a statistically significant Prime Type × Target Type interaction at the medial-lateral sites, F(1, 34) = 4.83, p = .035, η2 = .12. Moreover, there was a significant Prime Type × Target Type × Hemisphere × Electrode interaction at the medial-lateral sites, F(1, 34) = 5.88, p = .02, η2 = .15, and a significant Prime Type × Target Type × Hemisphere interaction at the lateral-lateral sites, F(1, 34) = 4.14, p = .05, η2 = .11. Planned contrasts at each electrode site showed that the Prime Type × Target Type interaction was significant in the posterior sites of the right hemisphere, specifically, Pz, F4, C4, P4, P8, and O2. These findings suggest that the N400 effect (i.e., greater N400 amplitude for rejection than for acceptance cues) was greater after attachment-relevant primes than after nonattachment-relevant primes.
Finally, to assess whether these results reflected activity throughout the entire 250- to 600-ms epoch, we created successive 50-ms epochs during this interval and ran a separate ANOVA on each 50-ms epoch (Grainger, Kiyonaga, & Holcomb, 2006). The results for individual 50-ms epochs were highly consistent with the results for the entire 250- to 600-ms epoch (see Table 1).
TABLE 1.
Modulation of N400 Amplitudes in Individual 50-Ms Epochs as a Function of Prime Type, Target Type, Women's Adult Attachment, and Electrode Site
| Epoch (ms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Interaction | 250–600 | 250–300 | 300–350 | 350–400 | 400–450 | 450–500 | 500–550 | 550–600 |
| Midline electrodes | ||||||||
| P × T | < .10 | > .10 | > .10 | > .10 | < .05 | > .10 | < .05 | < .05 |
| P × T × E | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 |
| Medial-lateral electrodes |
||||||||
| P × T | < .05 | > .10 | > .10 | < .10 | < .05 | < .05 | < .05 | < .05 |
| P × T × E | < .05 | < .01 | < .001 | < .01 | < .05 | < .10 | < .05 | > .10 |
| P × T × H | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | < .10 | > .10 |
| P × T × E × H | < .05 | > .10 | < .05 | > .10 | < .05 | < .05 | < .01 | < .10 |
| Lateral-lateral electrodes |
||||||||
| P × T | > .10 | > .10 | > .10 | > .10 | < .10 | > .10 | < .10 | < .10 |
| P × T × E | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 |
| P × T × H | < .05 | > .10 | > .10 | < .10 | < .05 | > .10 | < .10 | > .10 |
| P × T × E × H | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 | > .10 |
| Right-hemisphere centroparietal sites (Cz, Pz, C4, P4) |
||||||||
| P × T × Anxiety × Avoidance | < .05 | < .01 | < .01 | > .10 | < .10 | > .10 | > .10 | < .10 |
| T × Anxiety × Avoidancea | < .05 | < .05 | < .01 | < .05 | < .10 | < .05 | < .10 | > .10 |
Note. The table reports p values produced from a series of general linear models examining the effects of prime type (P; attachment vs. nonattachment), target type (T; acceptance vs. rejection), electrode (E), and hemisphere (H) on event-related potential (ERP) amplitudes during the entire 250- to 600-ms epoch and individual 50-ms epochs during this window. Boldface highlights p values less than .10. The results for the individual 50-ms epochs were highly consistent with those for the entire 250- to 600-ms epoch.
The p values in this row are from analysis of the attachment-prime condition only.
N400 Amplitudes and Individual Differences in Adult Attachment
Do individual differences in N400 amplitude in response to partner rejection cues relate meaningfully to women’s adult attachment? To answer this question, we computed the mean N400 amplitude recorded at the centroparietal electrodes of the right hemisphere (Cz, C4, Pz, P4), which show especially strong N400 effects (Kutas & Hillyard, 1980, 1982; Kutas et al., 1988). This mean was entered as the outcome variable in a general linear model (GLM) with prime type (attachment vs. nonattachment) and target type (rejection vs. acceptance) as within-subjects factors, and mean-centered avoidance, mean-centered anxiety, and the Avoidance × Anxiety interaction term as continuous between-subjects factors.
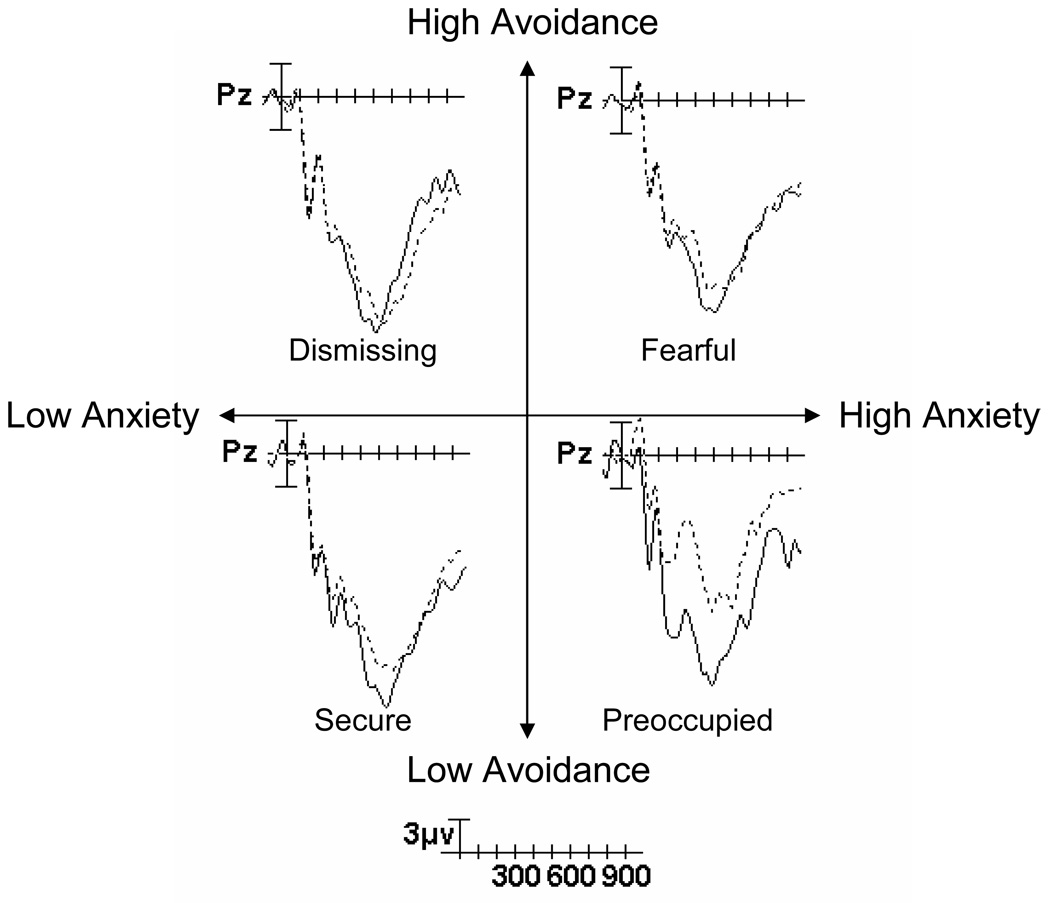
Individual differences in women’s adult attachment significantly modulated N400 responses. Figure 2 shows the grand-averaged ERP waveforms recorded at Pz after attachment-relevant primes as a function of target type (acceptance vs. rejection) and adult attachment. The four-way Anxiety × Avoidance × Prime Type × Target Type interaction was statistically significant, F(1, 31) = 4.09, p = .05, η2 = .12. Follow-up tests indicated a significant Anxiety × Avoidance × Target Type interaction, F(1, 31) = 6.60, p = .02, η2 = .18, as well as an Anxiety × Target Type interaction, F(1, 31) = 5.81, p = .02, η2 = .16, and an Avoidance × Target Type interaction, F(1, 31) = 5.66, p = .02, η2 = .15. The avoidance and anxiety dimensions did not predict N400 amplitudes in the nonattachment condition (all ps for main effects and interactions were nonsignificant).
Fig. 2.
Grand-average event-related potential waveforms recorded at Pz after attachment-related primes as a function of target type (acceptance vs. rejection) and general adult attachment. Negative voltages are plotted upward. Median splits were used to assign participants into high- and low-avoidance groups and high- and low-anxiety groups. Because scores on the anxiety and avoidance scales can be combined to assign individuals to four categories of adult attachment style (secure, preoccupied, dismissing, and fearful), these labels are also shown.
To examine the Anxiety × Avoidance × Target Type interaction in the attachment condition, we performed simple-slopes analyses (Aiken & West, 1991). Among women high in anxiety (1 SD above the mean), low levels of avoidance were associated with magnified attachment-related N400 effects, β = −.90, t (30) = −3.55, p = .001, whereas among women low in anxiety (1 SD below the mean), avoidance was unrelated to N400 effects, β = .19, t (30) = 0.72, p = .48. Results from analyses conducted on individual 50-ms epochs were highly consistent with those for the entire 250- to 600-ms epoch (see Table 1).
Behavioral Data
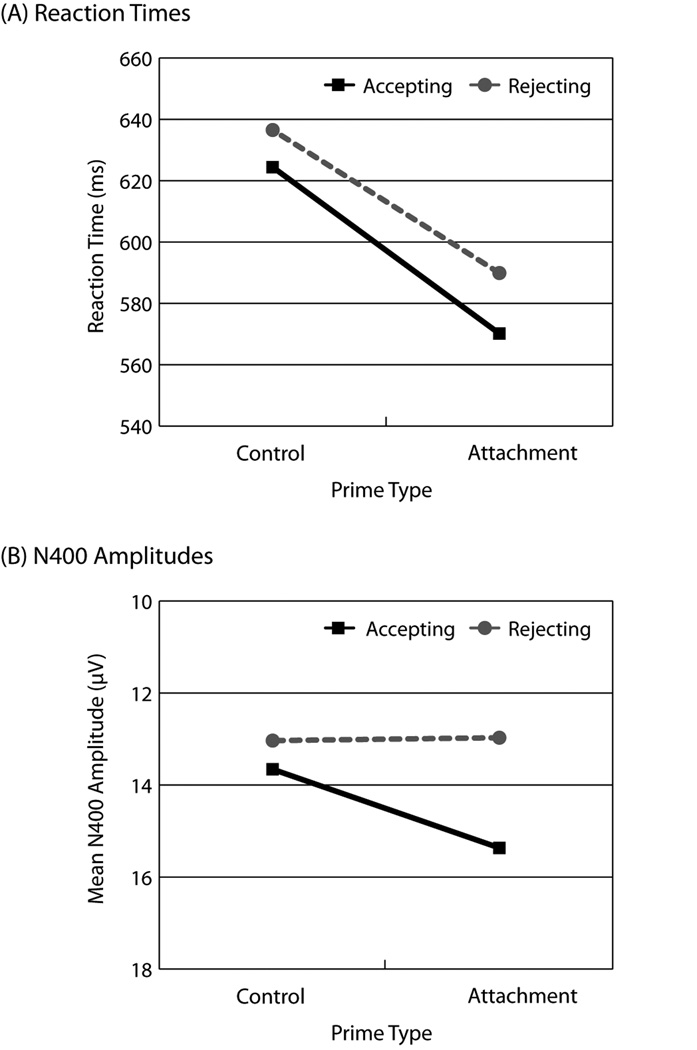
Behavioral results replicated previous behavioral findings (Baldwin et al., 1993; see Fig. 3a). Interpersonal target words, regardless of valence, were classified more quickly within attachment contexts than within nonattachment contexts, F(1, 34) = 31.23, p < 10−5, η2 = .48. In addition, acceptance targets were classified more quickly than rejection targets, F(1, 34) = 76.76, p < 10−9, η2 = .69. The Prime Type × Target Type interaction was not statistically significant.
Fig. 3.
Comparison of behavioral responses (reaction time) and neurophysiological responses (N400). The graphs show (a) mean reaction time and (b) mean N400 amplitudes (within the 250- to 600-ms window) recorded at right-hemisphere centroparietal electrode sites (i.e., Cz, C4, Pz, and P4) as a function of prime type (attachment vs. nonattachment) and target type (acceptance vs. rejection). Values on the y-axis have been reversed so that more negative voltages are plotted upward.
We used GLMs to examine the relations between adult attachment and RTs on the LDT. Compared with women who were low in avoidance, women high in avoidance were slower at classifying rejection words, relative to acceptance words, in the attachment condition; the Avoidance × Target Type interaction was significant, F(1, 31) = 4.98, p = .03, η2 = .14. In contrast, the Avoidance × Target Type interaction was not significant in the nonattachment condition, F(1, 31) = 0.39, p = .54, η2 = .01. Women high in anxiety, compared with women low in anxiety, were faster at classifying rejection words, relative to acceptance words, regardless of the preceding prime, F(1, 31) = 3.74, p = .06, η2 = .11.
Thus, avoidance was related not only to dampened attachment-related N400 effects, but also to slower RTs to rejection cues encountered after attachment primes—a pattern that suggests disengagement from threat-relevant information. In contrast, anxiety, which was related to enhanced attachment-related N400 effects, was associated with faster RTs to partner rejection cues (though this effect was not specific to performance following attachment primes)—a pattern indicating greater processing as indexed by the N400 and also quicker identification of threat-relevant information.
DISCUSSION
We examined early-stage processing of partner rejection cues using ERPs, focusing on an ERP component (N400) that has previously been linked with semantic processing. When primed with a situation designed to activate attachment-relevant thoughts and feelings, participants exhibited larger N400 amplitudes in response to rejection words than in response to acceptance words. Moreover, differences in amplitude emerged as early as 250 to 300 ms, indicating that the brain differentially responds to partner rejection (vs. acceptance) cues in less than 300 ms.
These early-stage neurophysiological responses were meaningfully related to women’s adult attachment. Women characterized by a combination of high attachment anxiety and low attachment avoidance showed the greatest N400 responses to partner rejection cues. This suggests that the effect of partner rejection cues on neural responses depends on one’s sensitivity to partner rejection (captured by the anxiety dimension) and on the extent to which one engages with (“moves toward,” or approaches) versus disengages from (“moves away from,” or avoids) the source of the initial threat (captured by the avoidance dimension). This research is the first to show that individual differences in adult attachment are reflected in neurophysiological responses as early as 300 ms after partner rejection cues are encountered.
These findings are in line with current thinking that the N400 amplitude indexes the total amount of semantic processing prompted by a cue. One possible factor that may have prompted increased processing is the unexpectedness of partner rejection, which might cause a “second look.” This possibility is consistent with research showing that unexpected outcomes produce greater N400 amplitudes than expected outcomes (Kutas & Hillyard, 1984) and that false statements produce greater N400 amplitudes than true statements (Hagoort et al., 2004). However, past research suggests that other factors also influence N400 amplitude. For example, research has shown that the basic expectation-violation effect is amplified when attention is directed to the stimulus stream (Bentin et al., 1995; Kiefer & Brendel, 2006).
The present results support the idea that the N400 reflects total amount of semantic processing, not simply expectation violation. First, the behavioral data (Fig. 3a) suggest that partner rejection may not be entirely unexpected. Compared with nonattachment primes, attachment primes decreased RTs for classifying rejection cues, t (34) = 2.48, p = .02, d = 0.42, which suggests that attachment primes increase the accessibility of rejection cues. If expectation violation were the only factor influencing N400 amplitude, then these behavioral data would lead to the prediction that, compared with nonattachment primes, attachment primes would reduce N400 responses to rejection cues. However, the magnitude of N400 responses to rejection cues was high in the attachment condition (Fig. 3b), even though attachment primes reduced the N400 magnitude for acceptance cues, as reflected in a significant Prime Type × Target Type interaction, F(1, 34) = 5.13, p = .03, η2 = .13. Thus, although the concept of partner rejection may be accessible (as reflected by RTs), its personal significance and the threat it poses to the self may enhance semantic analyses (as reflected by ERPs).
Second, the data regarding individual differences also suggest that N400 amplitudes reflect total amount of semantic processing. If expectation violation were the only factor, then women with high attachment anxiety and low attachment avoidance, who are expected to be hypervigilant for partner rejection cues, would have shown dampened attachment-related N400 effects. Instead, they showed magnified responses. Moreover, greater anxiety was related to faster RTs in classifying rejection words. Thus, the results suggest that individuals with high attachment anxiety were quicker to identify threat and engaged in greater processing of that threat, compared with individuals with low attachment anxiety. Avoidance, in contrast, was associated with dampened attachment-related N400 responses and slower RTs to rejection cues after attachment primes; this pattern of results suggests that avoidant individuals disengaged from the threat.
The inclusion of nonattachment primes in this study rules out two alternative explanations. First, the results cannot be attributed simply to the valence of the target. If that were the case, then rejection words, regardless of the preceding prime, should have been analyzed to a greater extent than acceptance words. Second, the results are not simply attributable to the strength of lexical associations between words in the primes and the subsequently presented targets. According to a lexical-association account, the mere presentation of primes containing self- and partner-relevant words, which are both associated with positive valence (Zayas & Shoda, 2005), should facilitate the processing of acceptance, relative to rejection, targets. However, self- and partner-referent words were present in both the attachment-relevant and the nonattachment primes. Thus, the results indicate that the N400 is sensitive not only to the properties of the target, which were the same in the two priming conditions, but also to the context in which the target occurs.
Would these effects occur if the cues involved rejection by unknown people or by people one would like to be close to? On the one hand, because romantic partners serve special roles as attachment figures, partner rejection cues are likely to be processed to a greater extent than cues of rejection by other people. On the other hand, individual differences in adult attachment are related to the processing of valenced stimuli more generally (Zilber et al., 2007), and one can feel rejected even by people one does not know. Thus, future research is needed to determine the extent to which the present findings generalize to other individuals.
Finally, although a general attachment orientation predicted attachment-related N400 responses, adult attachment with regard to participants' specific romantic relationships did not (see footnote 1). (The difference between the general and the specific ECR in predicting N400 responses was not statistically significant.) However, this was likely due to the low power in detecting the necessary five-way interaction.) The results may reflect differences between general and specific measures of attachment. General measures may tap into chronic, well-rehearsed processing dynamics, whereas partner-specific measures may be influenced by the particulars of a relationship and may tap into contextual, less-rehearsed processing dynamics.
This research highlights the utility of using ERPs for elucidating processing dynamics that may not be observable via behavioral measures alone. The results show that the brain responds differentially to cues of partner rejection versus acceptance in under 300 ms, and that individual differences in this response emerge as early as 300 ms after the onset of rejection cues.
Acknowledgments
This study was supported in part by Grant MH39349 from the National Institute of Mental Health and Grant DC01947 from the National Institute for Deafness and Other Communication Disorders. We are grateful to Peter Mullen, Aaron Maxwell, and David Grayson.
Footnotes
Participants also completed the Rejection Sensitivity Questionnaire (RSQ; Downey & Feldman, 1996) and a partner-specific ECR questionnaire. The results for the RSQ were similar to those reported here for the ECR attachment-anxiety scale. Scores on the partner-specific ECR were not meaningfully related to N400 responses. For all the analyses (behavioral and ERP), statistically controlling for relationship length produced results that are highly consistent with those reported here.
REFERENCES
- Aiken LS, West SG. Newbury Park, CA: Sage; 1991. Multiple regression: Testing and interpreting interactions. [Google Scholar]
- Ayduk O, Downey G, Kim M. Rejection sensitivity and depressive symptoms in women. Personality and Social Psychology Bulletin. 2001;27:868–877. [Google Scholar]
- Ayduk O, Downey G, Testa A, Yen Y, Shoda Y. Does rejection elicit hostility in high rejection sensitive women? Social Cognition. 1999;17:245–271. [Google Scholar]
- Baldwin MW, Fehr B, Keedian E, Seidel M, Thomson D. An exploration of the relational schemata underlying attachment styles: Self-report and lexical decision approaches. Personality and Social Psychology Bulletin. 1993;19:746–754. [Google Scholar]
- Bentin S, Kutas M, Hillyard S. Semantic processing and memory for attended and unattended words in dichotic listening: Behavioural and electrophysiological evidence. Journal of Experimental Psychology: Human Performance. 1995;21:54–67. doi: 10.1037//0096-1523.21.1.54. [DOI] [PubMed] [Google Scholar]
- Bowlby J. New York: Basic Books; Attachment and loss: Separation. [Google Scholar]
- Brennan KA, Clark CL, Shaver PR. Self-report measurement of adult romantic attachment: An integrative overview. In: Simpson JA, Rhodes WS, editors. Attachment theory and close relationships. New York: Guilford Press; 1998. pp. 46–76. [Google Scholar]
- Collins NL, Feeney BC. A safe haven: An attachment theory perspective on support-seeking and caregiving in adult romantic relationships. Journal of Personality and Social Psychology. 2000;78:1053–1073. doi: 10.1037//0022-3514.78.6.1053. [DOI] [PubMed] [Google Scholar]
- Compton RJ. The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews. 2003;2:115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;2:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dewitte M, Koster EHW, De Houwer J, Buysse A. Attentive processing of threat and adult attachment: A dot-probe study. Behaviour Research and Therapy. 2007;45:1307–1317. doi: 10.1016/j.brat.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70:1327–1343. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Downey G, Mougios V, Ayduk O, London B, Shoda Y. Rejection sensitivity and the defensive motivational system: Insights from the startle response to rejection cues. Psychological Science. 2004;15:668–673. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Shaver PR. Adult attachment and the suppression of unwanted thoughts. Journal of Personality and Social Psychology. 1997;73:1080–1091. doi: 10.1037//0022-3514.73.5.1080. [DOI] [PubMed] [Google Scholar]
- Grainger J, Kiyonaga K, Holcomb P. The time course of orthographic and phonological code activation. Psychological Science. 2006;17:1021–1026. doi: 10.1111/j.1467-9280.2006.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Hald L, Bastiaansen MCM, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304:438–440. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver PR. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Brendel D. Attentional modulation of unconscious “automatic” processes: Evidence from event-related potentials in a masked priming paradigm. Journal of Cognitive Neuroscience. 2006;18:184–198. doi: 10.1162/089892906775783688. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. The lateral distribution of event-related potentials during sentence processing. Neuropsychologia. 1982;20:579–590. doi: 10.1016/0028-3932(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C, Besson M. Event-related potential asymmetries during the reading of sentences. Electroencephalography and Clinical Neurophysiology. 1988;69:218–233. doi: 10.1016/0013-4694(88)90131-9. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C, Kluender R. Psycholinguistics electrified II (1999–2005) In: Traxler MJ, Gernsbacher MA, editors. The handbook of psycholinguistics. 2nd ed. London: Elsevier; 2006. pp. 659–724. [Google Scholar]
- Mikulincer M. Adult attachment style and individual differences in functional versus dysfunctional experiences of anger. Journal of Personality and Social Psychology. 1998;74:513–524. doi: 10.1037//0022-3514.74.2.513. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Birnbaum G, Woddis D, Nachmias O. Stress and accessibility of proximity-related thoughts: Exploring the normative and intraindividual components of attachment theory. Journal of Personality and Social Psychology. 2000;78:509–523. doi: 10.1037//0022-3514.78.3.509. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Florian V. Appraisal and coping with a real-life stressful situation: The contribution of attachment styles. Personality and Social Psychology Bulletin. 1995;21:408–416. [Google Scholar]
- Mikulincer M, Gillath O, Shaver PR. Activation of the attachment system in adulthood: Threat-related primes increase the accessibility of mental representations of attachment figures. Journal of Personality and Social Psychology. 2002;83:881–895. [PubMed] [Google Scholar]
- Mikulincer M, Orbach I. Attachment styles and repressive defensiveness: The accessibility and architecture of affective memories. Journal of Personality and Social Psychology. 1995;68:917–925. doi: 10.1037//0022-3514.68.5.917. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment patterns in adulthood: Structure, dynamics, and change. New York: Guilford Press; 2007. [Google Scholar]
- Neely JH. Semantic priming and retrieval from lexical memory: Roles of inhibitionless spreading activation and limited-category attention. Journal of Experimental Psychology: General. 1977;106:226–254. [Google Scholar]
- Pizzagalli D, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: An ERP study. NeuroReport. 1999;10:2691–2698. doi: 10.1097/00001756-199909090-00001. [DOI] [PubMed] [Google Scholar]
- Powers S, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples’ attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. Journal of Personality and Social Psychology. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Radecki-Bush C, Farrell AD, Bush JP. Predicting jealous response: The influence of adult attachment and depression on threat appraisal. Journal of Social and Personal Relationships. 1993;10:569–588. [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation and reactions in youngsters: A transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Shaver PR, Hazan C. Adult romantic attachment: Theory and evidence. In: Perlman D, Jones WH, editors. Advances in personal relationships. Vol. 4. London: Jessica Kingsley; 1993. pp. 29–70. [Google Scholar]
- Simpson JA, Rhodes WS, Nelligan JS. Support seeking and support giving within couples in an anxiety-provoking situation: The role of attachment styles. Journal of Personality and Social Psychology. 1992;62:434–446. [Google Scholar]
- Zayas V, Shoda Y. Do automatic reactions elicited by thoughts of romantic partner, mother, and self relate to adult romantic attachment? Personality and Social Psychology Bulletin. 2005;31:1011–1025. doi: 10.1177/0146167204274100. [DOI] [PubMed] [Google Scholar]
- Zilber A, Goldstein A, Mikulincer M. Adult attachment orientations and the processing of emotional pictures: ERP correlates. Personality and Individual Differences. 2007;43:1898–1907. [Google Scholar]