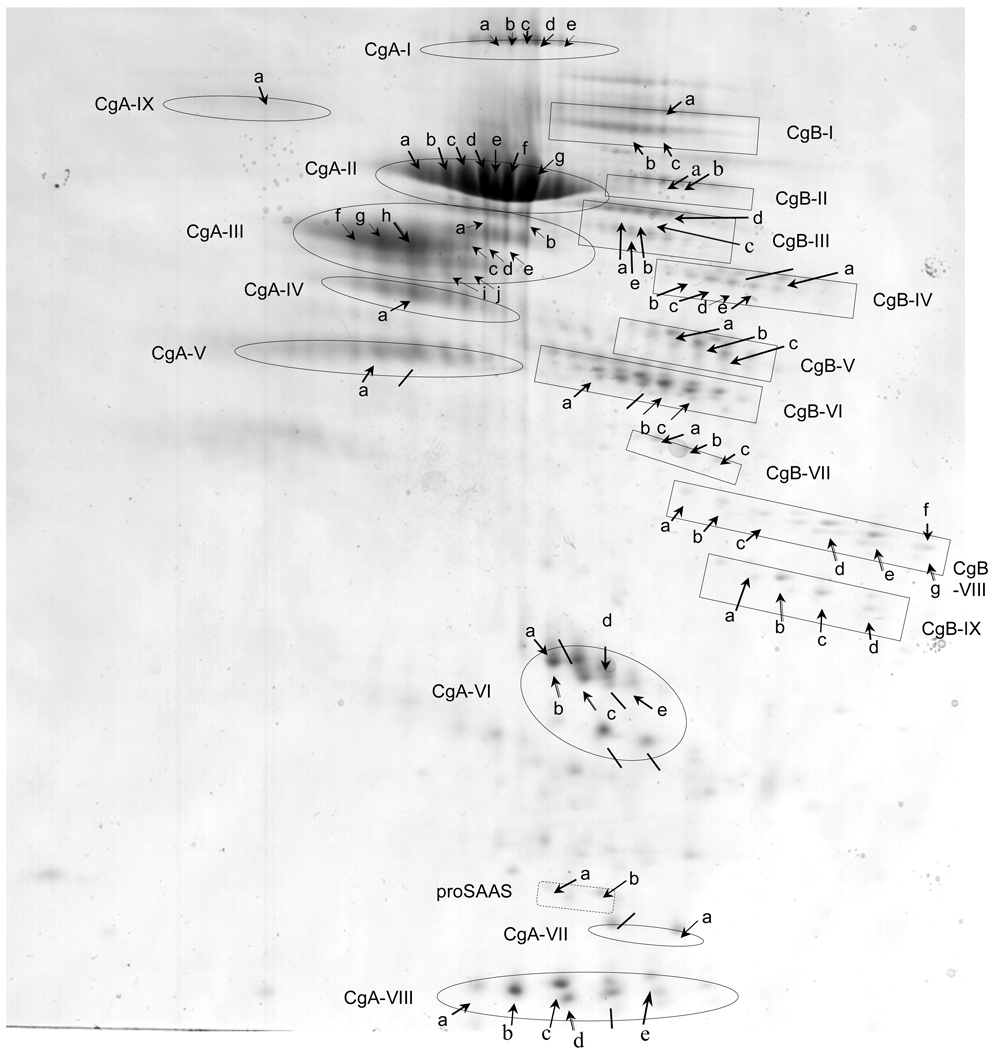
Figure 5. Series of chromaffin granule protein spots from 2-D gels subjected to analyses by MALDI-TOF mass spectrometry.
Two-dimensional gels of the soluble fraction of purified chromaffin granules yielded numerous protein spots (indicated by arrows) which were subjected to analyses by MALDI-TOF mass spectrometry after excision and trypsin digestion. Proteins that were identified by both MALDI-TOF MS and N-terminal sequencing are listed: CgA-I-(d), CgA-II-(e, g), CgA-III-(h), CgA-IV-(a), CgA-VI-(b, c), CgA-VII-(a), CgA-VIII-(b, c, d), CgB-III-(c), CgB-VI-(b, c), CgB-VIII-(d, e, g), CgB-IX-(b, c), proSAAS-(b). Also, proteins that were identified by N-terminal sequencing only, are indicated by lines (from fig. 1). MALDI-TOF results (see supplemental Table A, supplemental Table B, and supplemental Table C) showed that most of these spots represented CgA and CgB, while two minor spots represented proSAAS (supplemental Table C). Proteins of similar apparent molecular weight but possessing a range of isoelectric points (pI) are grouped, and indicated as proteins groups CgA-I to CgA-IX, and CgB-I to CgB-IX, and proSAAS (a,b). These data demonstrate the preponderance of CgA- and CgB-derived proteolytic fragments in the soluble fraction of chromaffin granules.