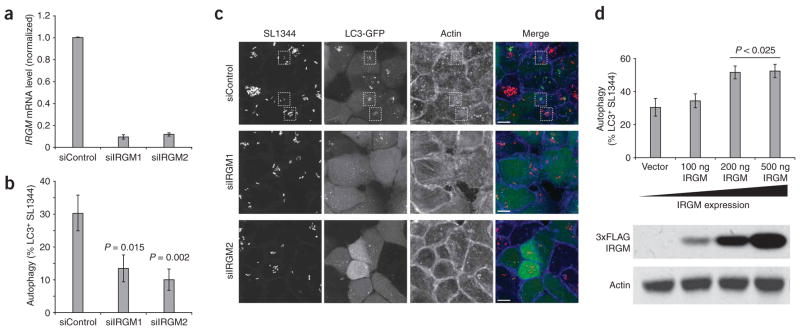
Figure 3.
IRGM expression levels affect the autophagy of Salmonella typhimurium in human epithelial cells. (a) siRNA constructs directed at IRGM reduced endogenous IRGM transcripts by six- to eightfold in HeLa cells. Cells were transfected with control (siControl) or IRGM-targeted (siIRGM1, siIRGM2) siRNA duplexes and assayed 48 h later by quantitative RT-PCR. Error bars, s.d. (b) IRGM knockdown reduces the efficiency of anti-bacterial autophagy. HeLa cells stably expressing LC3-GFP (a marker for autophagic vesicles) were transfected with control or IRGM-directed siRNA oligos, then infected with S. typhimurium after 48 h. The percentage of bacteria encapsulated in LC3-positive vesicles was determined by microscopy. Reductions in IRGM expression resulted in a significant loss of anti-bacterial autophagy compared to control cells. (c) Microscopic examination reveals a decreased number of autophagically encapsulated bacteria in IRGM knockdown cells. Control siRNA-treated (siControl) and IRGM siRNA-treated (siIRGM1, siIRGM2) cells were infected with S. typhimurium SL1344 and imaged by confocal microscopy. Control cells show numerous bacteria (red in merged image) surrounded by LC3-GFP membranes (green in merged image). Such bacteria-containing autophagosomes (indicated by dashed boxes) were almost completely absent in IRGM-deficient cells. The actin cytoskeleton was visualized using phalloidin (blue in merge). Images are flat projections of confocal z-stacks. Scale bars, 10 μm. (d) HeLa cells stably expressing LC3-GFP were transfected with increasing amounts of an IRGM expression construct and infected 24 h later with S. typhimurium. The percentage of bacteria found within LC3-positive vesicles increased with increasing amounts of exogenous IRGM. Protein expression levels were confirmed by blotting lysates for Flag-IRGM expression, using an antibody to Flag to detect exogenous IRGM and actin as a loading control (lower panel). Error bars in b and d, s.e.m.