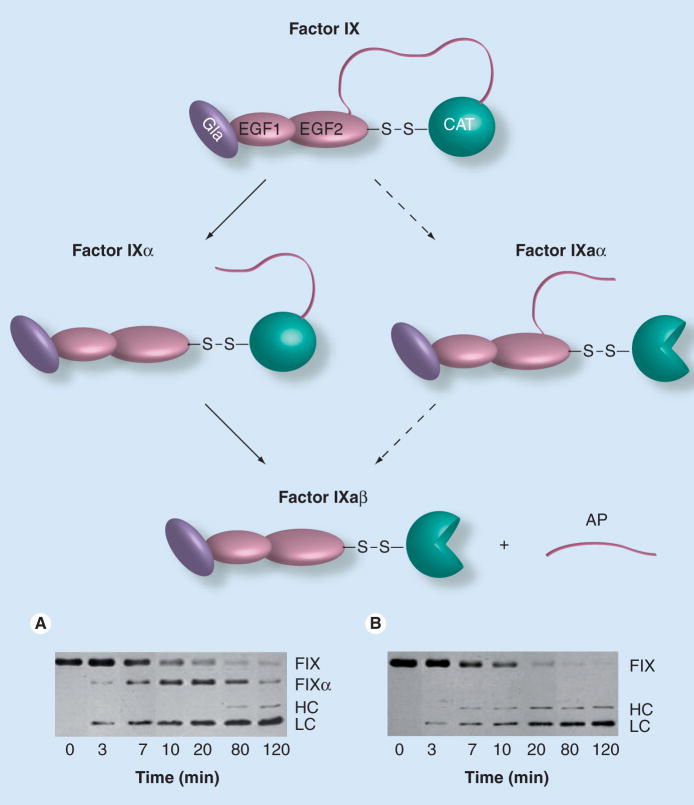
Figure 3. Factor IX activation.
Possible pathways involved in factor IX activation to the active protease factor IXaβ (top) and two western immunoblots of time courses of factor IX activation by (A) factor VIIa/tissue factor (TF) or (B) factor XIa. Factor IX is converted to factor IXaβ by cleavage after Arg145 and Arg180, releasing the activation peptide (the ribbon connecting EGF2 to CAT) that connects the CAT domain, also known as the heavy chain, to the noncatalytic light chain (Gla and EGF domains). The heavy chain and light chains of factor IXaβ remain attached by a disulfide bond. Factor VIIa/TF initially cleaves factor IX after Arg145, forming the intermediate factor IXα (A), which is not an active enzyme because the activation peptide remains attached to the catalytic domain. Factor VIIa/TF subsequently cleaves factor IXα after Arg180 to form factor IXaβ. The active protease domain of factor IXaβ is indicated by the three-quarter circle. Factor XIa also cleaves factor IX initially after Arg145, however, no intermediate is observed in time-course experiments (B) because the subsequent cleavage after Arg180 must be relatively rapid. Initial cleavage of factor IX after Arg180 to form the partially active intermediate factor IXaα is a minor reaction during normal coagulation, but is the preferred pathway of activation by a protease found in the venom of Russell’s viper (Daboia russelli). The western blots in (A & B) are of reduced factor IX proteins. The abbreviations to the right of each panel indicate the positions of uncleaved factor IX (FIX), the large chain of factor IXα that is comprised of the catalytic domain and activation peptide (FIXα), the heavy chain (HC) of factor IXaβ that is the active catalytic domain, and the light chain (LC – the Gla and EGF domains) that is a component of both factor IXα and factor IXaβ.
CAT: Catalytic; GLA: γ-carboxy glutamic acid; TF: Tissue factor.