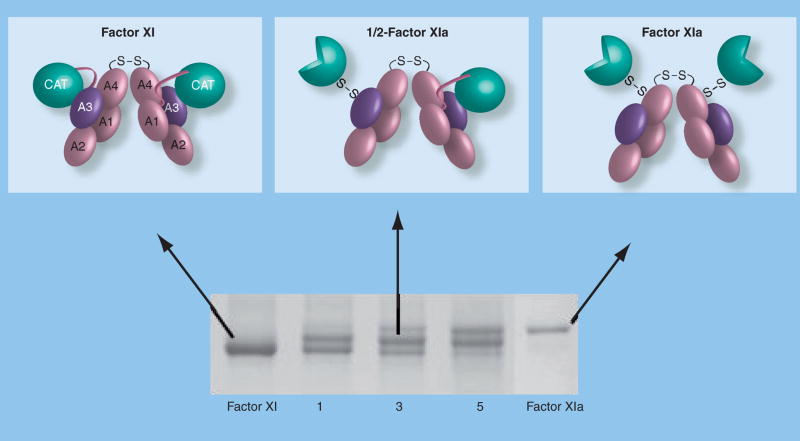
Figure 4. Activation of factor XI.
Sequential activation of factor XI subunits (top) and an SDS-polyacrylamide gel of a time course of factor XI activation by α-thrombin. The identical 80-kDa subunits of the factor XI dimer interface with each other through the A4 domains. A disulfide bond forms between Cys321 on each A4 domain. Each factor XI subunit is activated by cleavage after Arg369 between the A4 and catalytic domain. The resulting heavy chain (domains A1–A4) remains connected to the active catalytic domain (light chain – indicated by three-quarter circles) by a disulfide bond. Note on the 6% nonreducing polyacrylamide gel that factor XIa migrates through the gel slightly more slowly than factor XI. This is likely related to a conformational change associated with activation that exposes a factor IX-binding exosite on the A3 domain. The activation time course shown in the lower panel clearly demonstrates that conversion of factor XI to factor XIa proceeds through an intermediate that migrates between the substrate and final product. Purification and analysis of this intermediate demonstrated that it is a species with one cleaved subunit and one uncleaved subunit (1/2-FXIa).
A: Apple; CAT: Catalytic.