Figure 5. Functional analyses of differentiated hES cells enriched toward a hepatocyte phenotype.
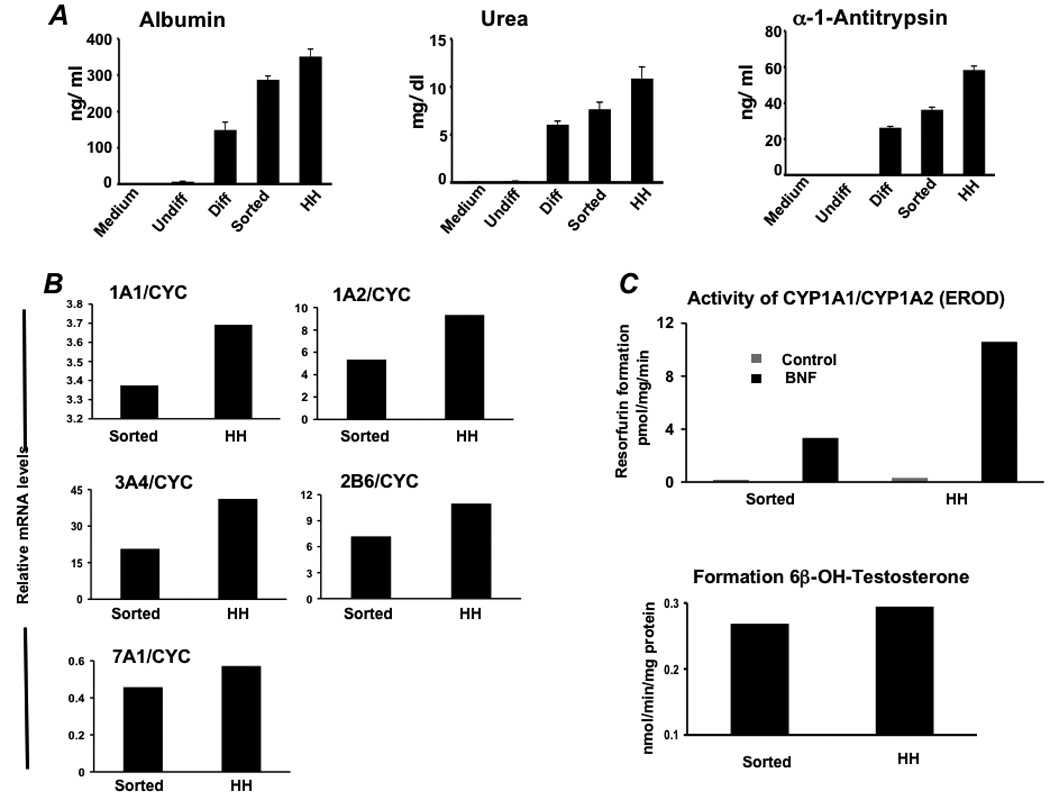
(A) Albumin, urea, and alpha-1-antitrypsin secretion was determined in vitro by primary human hepatocytes (HH) and hES cells during differentiation (n= 5). Analysis involved undifferentiated hES cells (Undiff), hES cells following EB formation (EBs), hES cells after treatment with FGF-2 and Activin-A or at the definitive endoderm (DE) stage, hES cells after culture in HGF and DMSO (early Diff), hES cells after culture in dexamethasone (Diff), and hES cells after the Diff stage and following enrichment for ASGPR surface expression. (B) Real time analysis demonstrated expression of cytochrome P450 1A1 (CYP1A1), 1A2, 3A4, 2B6, and 7A1 at levels similar to that derived from fresh primary human hepatocytes. (C) To assess human liver-specific cytochrome P450 metabolic activity, ES-derived- or normal human hepatocytes were cultured in the presence of phenobarbital or 25 µM BNF. For measurement of CYP 1A activity, cells were exposed to media containing 20 µM 7-ethoxyresorufin and conversion to 7-hydroxyresorufin in the media was quantified by the fluorescence of the 7-hydroxy metabolite measured at 535 nm (Ex) and 581 nm (Em). Analysis of CYP3A activity was measured by conversion of testosterone to 6β-hydroxytestosterone by high pressure liquid chromatography. Studies demonstrated BNF-inducible EROD activity at approximately 25–30% of that generated by primary human hepatocytes, and baseline formation of testosterone by differentiated ASGPR-enriched cells near that produced by cultured primary human hepatocytes.
