Table 2.
Isomerization of Allenamides at the α-Position.
| entry | allenamides | conditions [time]a | dienes | yield [%]b | E:Zc | ||
|---|---|---|---|---|---|---|---|
| 1 | 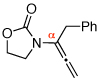 |
3 | 115 °C, 16 h | 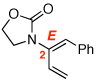 |
4 | 71 | 6:1 |
| 2 | 5a: R = n-Pr | 135 °C, 6 h | 8a | 77 | ≥20:1 | ||
| 3 | 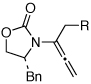 |
5a: R = n-Pr | CSA, 4 hd | 8a | 87 | ≥20:1 | |
| 4 | 5b: R = Ph | 135 °C, 16 h | 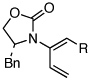 |
8b | 74 | ≥20:1 | |
| 5 | 5b: R = Ph | CSA, 2 h | 8b | 83 | ≥20:1 | ||
| 6 | 5c: R = 2Nap | 135 °C, 16 he | 8c | 73 | ≥50:1 | ||
| 7 | 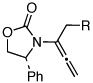 |
5d: R = H | 135 °C, 16 h | 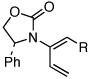 |
8d | 69 | - |
| 7 | 6a: R = n-Pr | CSA, 10 min | 9a | 82 | ≥50:1 | ||
| 8 | 6b: R = Ph | CSA, 10 min | 9b | 76 | ≥50:1 | ||
| 9 | 6c: R = H | 135 °C, 16 h | 9c | 69 | - | ||
| 10 | 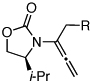 |
7a: R = n-Pr | 135 °C, 16 h | 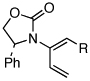 |
10a | 62 | ≥50:1 |
| 11 | 7b: R = Ph | 135 °C, 16 h | 10b | 82 | ≥50:1 | ||
| 12 | 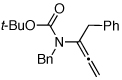 |
11 | 135 °C, 16 h | 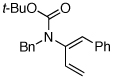 |
12 | 45 | ≥20:1 |
| 13 | 11 | CSA,f 2 h | 12 | 61 | ≥20:1 | ||
Unless otherwise noted, CH3CN was the solvent for thermal conditions, and CH2Cl2 was the solvent when using 10 mol % of CSA at rt. For all reactions, concn = 0.10 M.
Isolated yields.
Determined by 1H-NMR.
Temp started at −78 °C.
ClCH2CH2Cl was used.
4 Å MS was used.
