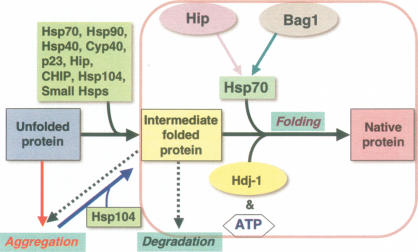
Figure 1.
Chaperone networks and the regulation of protein conformation. The unfolded protein transitions to the intermediate and native state assisted by molecular chaperones. The “holding” activity of chaperones is represented by formation of transient and stable intermediates that continue on-pathway to the native state upon interaction with an ATP-dependent chaperone machine such as Hsp70 (together with ATP and the cochaperones Bag1, Hip, and Hdj1). Unfolded proteins and intermediates can form aggregates that can be rescued by the Hsp104 class of chaperones. Likewise, intermediates can form aggregates, become degraded, or fold to the native state assisted by chaperones.