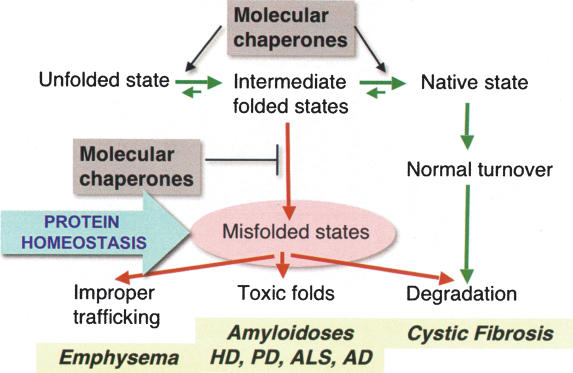
Figure 3.
Interplay between protein quality control (transition of unfolded to intermediates to native states) and clearance mechanisms in protein conformation disease. Chaperones have a critical role to suppress the appearance of misfolded species and to enhance protein folding. The imbalance of misfolded species is associated in human disease with premature clearance of CFTR as occurs in cystic fibrosis, to prevent improper trafficking of α-1-anti-trypsin as occurs in emphysema, and to prevent proteins from adopting toxic folds as in amyloidoses including huntingtin in Huntington’s disease, α-synuclein and parkin in Parkinson’s disease, mutant SOD1 in familial ALS, and Aβ in Alzheimer’s disease.