Table 1.
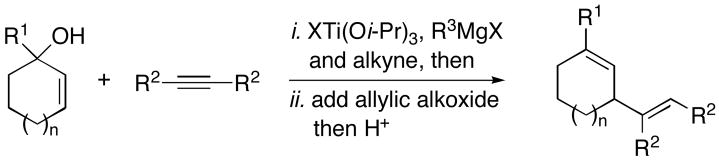 | ||||
|---|---|---|---|---|
| entry | allylic alcohol | alkyne | yield (%) | 1,4-dienea |
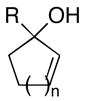
|
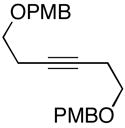
|
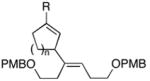
|
||
| 1 | 6; R = H, n=1 | 7 | 65 | 8; n=1 |
| 2 | 9; R = H, n=2 | 68 | 10; n=2 | |
| 3 | 11; R = Me, n=1 | 61 | 12; R = Me, n=1 | |
| 4 | 13; R = Ph, n=1 | 79 | 14; R = Ph, n=1 | |
| 5 | 15; R = c-C5H9, n=1 | 40 | 16; R = c-C5H9, n=1 | |
| 6 |
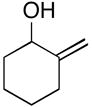
17 |
7 | 57 |
18 |
| 7b |
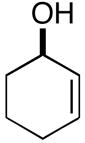
19(er = 97:3) |
7 | 54 |
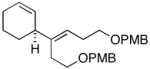
20c (er = 96:4) |
| 8 |
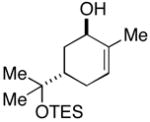
21 |
7 | 50 |
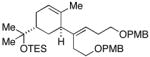
22(dr ≥ 20:1) |
Reaction conditions for cross coupling: alkyne (1.0 eq), ClTi(Oi-Pr)3, PhMe, C5H9MgCl, −78 to −35 °C, then recool to −78 °C, add Li-alkoxide of allylic alcohol (1.0 eq) (−78 to 0 °C).
ClTi(Oi-Pr)3 was replaced with Ti(Oi-Pr)4 in this experiment.
Absolute stereochemistry not determined.
