Table 2.
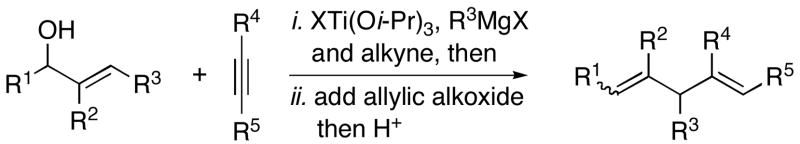 | |||||
|---|---|---|---|---|---|
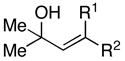
| entry | allylic alcohol | alkyne | yield (%) | E:Z | 1,4-dienea, b, c |
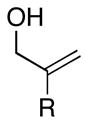
|
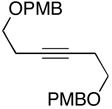
|
|
|||
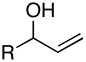
| 1 | 23; R = H | 7 | 41 | – | 24; R = H |
| 2 | 25; R = Me | 66 | – | 26; R = Me | |
| 3 |
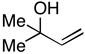
27 |
7 | 53 | – |
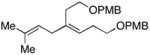
28 |
|
|
||||
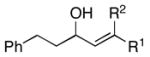
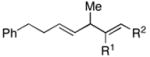
| 4 | 29; R1 =H, R2 =Me | 7 | 59 | – | 30 |
| 5 | 31; R1 =Me, R2 =H | 67 | – | ||
| 6 |
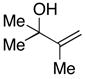
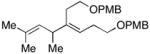
32 |
7 | 57 | – |
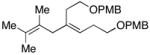
33 |
|
|
||||
| 7 | 34; R=Ph | 7 | 78 | 1:1 | 35; R=Ph (E/Z=1:1) |
| 8 | 36; R=Et | 67 | 1:1 | 37; R=Et (E/Z=1:1) | |
| 9 |
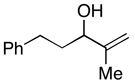
38 |
7 | 72 | ≥20:1 |
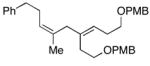
39 |
| 10 | 38 |
40 |
77 | ≥20:1 |
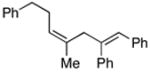
41d |
| 11 | 38 |
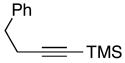
42 |
59 | ≥20:1 |
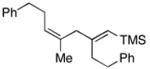
43e |
|
|
||||
| 12 | 44; R1=Me, R2=H | 7 | 67 | 1:1 | 45; R1, R2=(CH2)2OPMB |
| 13 | 46; R1=H, R2=Me | 42 | 59 | 8:1 | 47e; R1=(CH2)2Ph, R2=TMS |
| 14 |
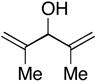
48 |
7 | 59 | ≥20:1 |
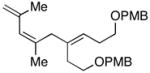
49 |
Reaction conditions for cross coupling: alkyne (1.0 eq), ClTi(Oi-Pr)3, PhMe, C5H9-MgCl, −78 to −35 °C, then recool to −78 °C, add Li-alkoxide of allylic alcohol (1.0 eq) (−78 to 0 °C).
All 1,4 diene products were isolated as a single olefin isomers.
No evidence was found for the production of regioisomeric products.
In the formation of the titanium–alkyne complex, the temperature was kept under −55 °C (see supporting information for details).
rr ≥ 20:1.
