Table 2.
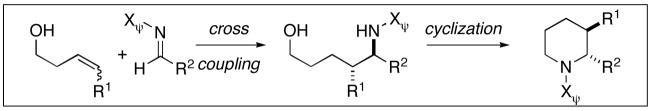 | ||||||
|---|---|---|---|---|---|---|
| entry | homoallylic alcohol | imine | 1,5-aminoalcohola | yield (%) | dr | piperidineb (yield) |
| 1 |
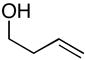 14 |
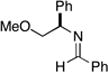 28 |
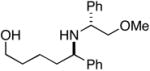 29 |
83 | 24:1 |
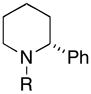 30 (77%) |
| 2 |
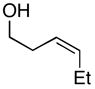 27 |
28 |
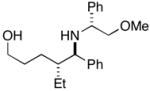 31 |
75 | 20:1 |
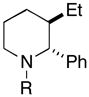 32 (87%) |
| 3 |
18 |
28 | 31 | 61 | 25:4:1 | |
Reaction conditions for cross coupling: imine (1 eq), Ti(Oi-Pr)4 (1.5 eq), c-C5H9MgCl (3.0 eq), Et2O (−60 to −30 °C), then add alkoxide (1.5 eq) (−40 to 0 °C or rt).
Reaction conditions for cyclization: For 30 - 2-NsCl, Et3N, DMAP, CH2Cl2, rt. For 32-MsCl, Et3N, CH2Cl2, rt. R = CH(Ph)CH2OMe.
