Table 3.
 | ||||||
|---|---|---|---|---|---|---|
| entry | homoallylic alcohol | imine | 1,5-aminoalcohola | yield (%) | dr | piperidineb (yield) |
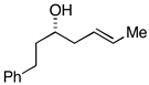
| 1 |
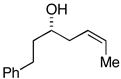 33 |
ent-28 |
 34c |
76 | ≥50:1 |
 35 (79%) |
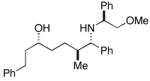
| 2a |
 33 |
28 |
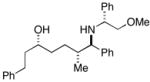 36c |
30 | 4:1:1 | |
| 2b | 85 | 6:3:1 | ||||
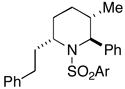
| 3 |
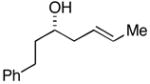 38 |
28 |
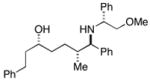 36c |
88 | 35:4:1 |
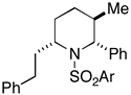 37(78%) |
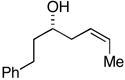
| 4 |
 38 |
ent-28 |
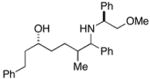 39 |
76 | 19:12:4:1 | |
Reaction conditions for cross coupling: imine (1 eq), Ti(Oi-Pr)4 (1.5 eq), c-C5H9MgCl (3.0 eq), Et2O (−60 to −30 °C), then add alkoxide (1.5 eq) (−30 to 0 °C (entries 1, 2a, 3 and 4) or rt (entry 2b)).
Reaction conditions for cyclization: 1) H2 (1 atm), Pd(OH)2, AcOH/MeOH (1:10 v/v), rt, 2) 2-NsCl, Et3N, DMAP, CH2Cl2, 0 °C to rt, 3) PPh3, DIAD, THF, rt. Ar = 2-nitrophenyl.
Major diastereomer shown.
Cyclization of 36 to 37 was performed with the enantiomer of 36 (for data on ent-37, see Supporitng Information).
