Table 2.
 | |||||
|---|---|---|---|---|---|
| entry | allylic alcohol | vinylsilane | yield (%)a | (Z:E) | product |
| 1 |
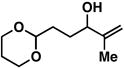 14 |
20 | 51 | ≥ 20:1 |
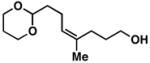 26 |
| 2 |
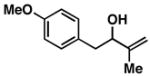 16 |
20 | 58 | ≥ 20:1 |
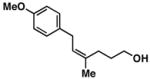 27 |
| 3 |
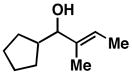 28 |
20 | 69 | ≥ 20:1 |
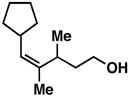 29 |
| 4 |
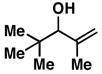 30 |
20 | 56 | ≥ 20:1 |
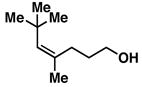 31 |
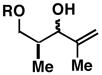
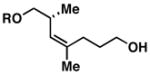
| 5 |
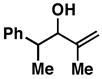 3211 |
20 | 64 | ≥ 20:1 |
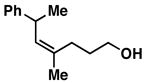 33 |
|
|
||||
| 6 | 34; R = TBS11 | 20 | 66 | ≥ 20:1 | 37; R = H |
| 7 | 35; R = TBDPS11 | 20 | 61 | ≥ 20:1 | 37; R = H |
| 8 | 36; R = PMB11 | 20 | 61 | 1.2:112 | 38; R = PMB |
Yield reported is over the two-step process: 1) Reductive cross-coupling (20, ClTi(Oi-Pr)3, c-C5H9MgCl, Et2O (−78 to −50 °C), then cool to −78 °C and add lithium alkoxide of the allylic alcohol (−78 to 0 °C) then, HCl (1N)), 2) oxidation (t-BuOOH, CsOH·H2O, TBAF, DMF, 70 °C).
